Interleukin-2 derivatives
A derivative, IL-2 technology, applied in the field of molecular biology, can solve the problems of ineffective treatment, cell apoptosis, toxic and side effects, etc., and achieve the effect of reducing VLS
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1I
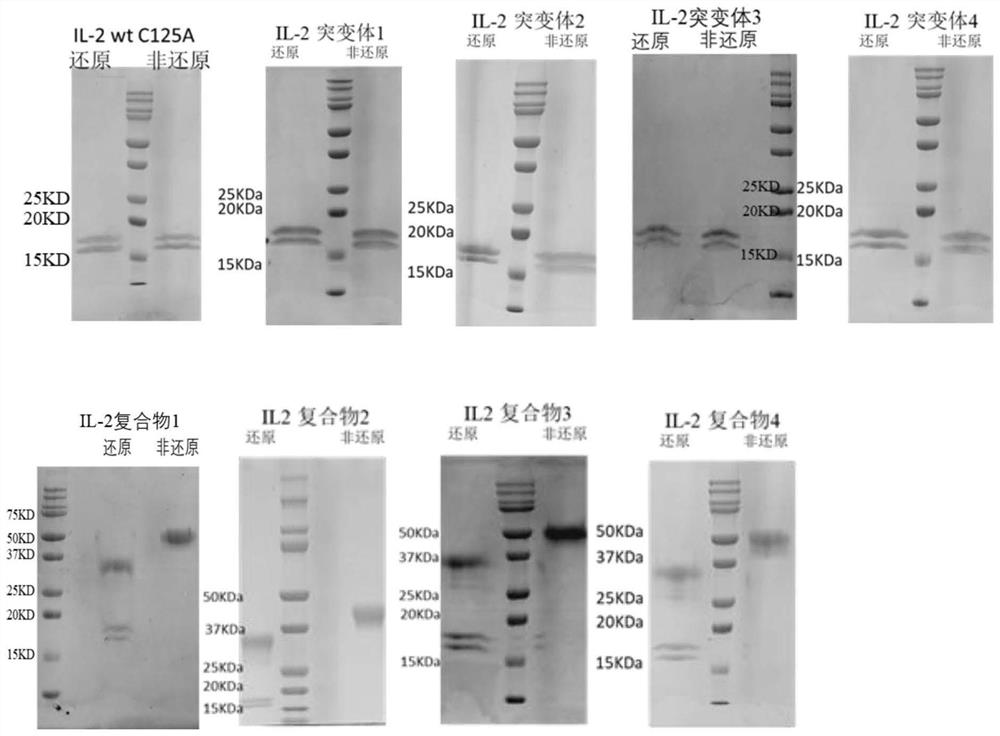
[0106] The preparation of embodiment 1IL-2 (C125A), IL-2 mutant and IL-2 complex
[0107] In this example, IL-2wt (C125A), IL-2 mutants 1-4 and IL-2 complexes 1-4 were selected for expression respectively, and were purified and prepared by relying on the HPC4 tag attached to the C-terminus of the molecule.
[0108] 1.1 Expression plasmid construction
[0109]Entrust Suzhou Jinweizhi Biotechnology Co., Ltd. to synthesize IL-2wt C125A (SEQ ID NO.74), IL-2 mutant 1-4, IL-2 complex 1-4 (IL-2Pair in IL-2 complex 1~4 and CD25-ECD Pair 1~4), and then perform overlapping PCR to obtain the target fragment according to the operation method mentioned in "Molecular Cloning". Then carry out the recombination connection of the fragment and the pTT5 universal vector, transform DH10B, sequence and bacterium preservation, so as to obtain the required IL-2wt (C125A), IL-2 mutant 1 ~ 4 and the plasmid of IL-2 complex 1 ~ 4 (IL Plasmids of IL-2Pair 1-4 and CD25-ECDPair 1-4 in the -2 complex); o...
Embodiment 2
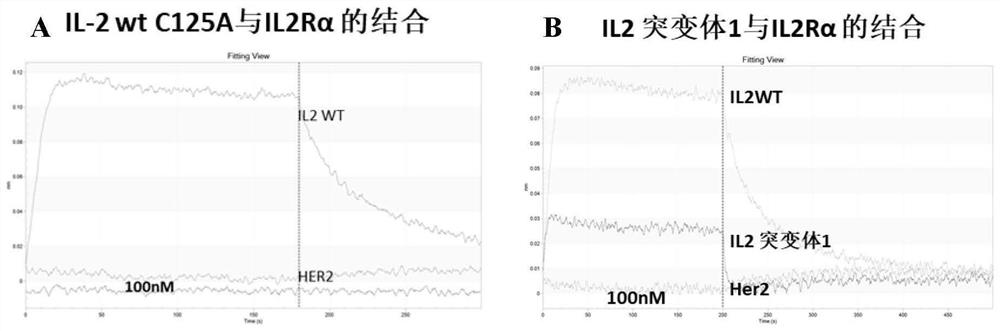
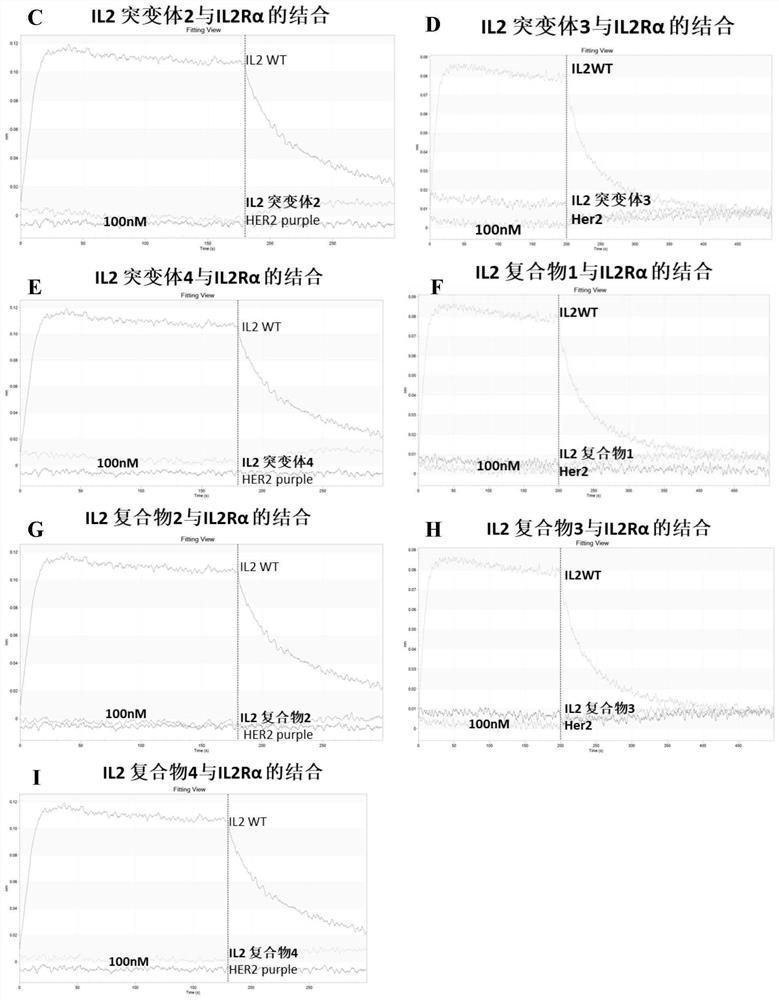
[0134] Example 2 Determination of the affinity of IL-2wt (C125A), IL-2 mutants 1-4 and IL-2 complexes 1-4 to IL2Rβγ and IL2Rα by biolayer interferomeory (BLI)
[0135] 1. Experimental materials
[0136] The proteins used in the experiment were all produced by Beijing Zhidao Biotechnology Co., Ltd. IL2Rα-his (purchased from Beijing Zhidao Biotechnology Co., Ltd.), IL2Rβγ-Fc (purchased from Beijing Zhidao Biotechnology Co., Ltd.) and IL2 mutants were transiently expressed by HEK293 and parent-child and obtained after purification. Buffer formulation: 10 mM HEPES, 150 mM NaCl, 3 mM EDTA, 0.1% BSA and 0.05% Tween 20; ProA sensor (Pall Fortebio, catalog #18-5010); HISIK sensor (Pall Fortebio, catalog #18- 5120); the BLI equipment was Octet RED96 produced by Pall Fortebio; data acquisition and analysis were performed using Data acquisition 11.0 and Data analysis 11.0 software, respectively.
[0137] 2. Experimental method
[0138] 1) Preparation of IL2Rβγ-Fc
[0139] Dilute IL2R...
Embodiment 3
[0152] Embodiment 3 promotes the proliferation experiment of T cell
[0153] The CTLL-2 (T cell) proliferation assay is a commonly used assay to measure the activity of interleukin-stimulated immune cells at the cell level. Therefore, the biological activity of IL-2 derivatives was examined here by the proliferation experiment of CTLL-2 cells.
[0154] 1) CTLL-2 cell preparation: the cells were resuspended in culture medium containing FBS and Rat-T-Stim.
[0155] 2) Adding samples: inoculate the cells in a 96-well culture plate, 0.1 ml per well. At the same time, the proteins of the IL-2 derivatives 11, 18, 21 and 28 of the samples to be tested (i.e. the proteins prepared in Example 1) were respectively diluted in multiples, and 0.1ml was added to each well, and each dilution concentration was set in 3 multiple wells. . Separately set up culture solution control wells (100ul cells + 100ul culture solution). 37 degrees, 5% CO 2 Incubate for 72 hours.
[0156] 3) Add MTS: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com