LOCKR-mediated CAR T cell recruitment
A cell and cell therapy technology, applied in the field of LOCKR-mediated CAR T cell recruitment, can solve problems such as difficult to re-use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0488] overview
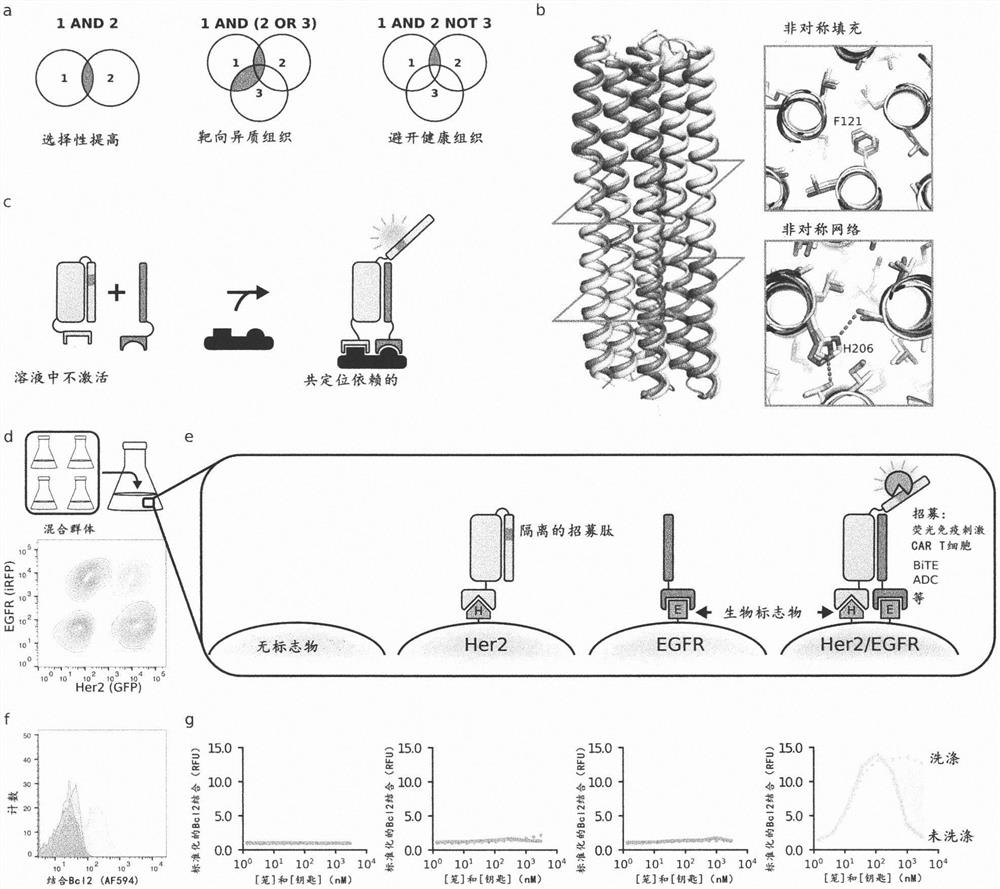
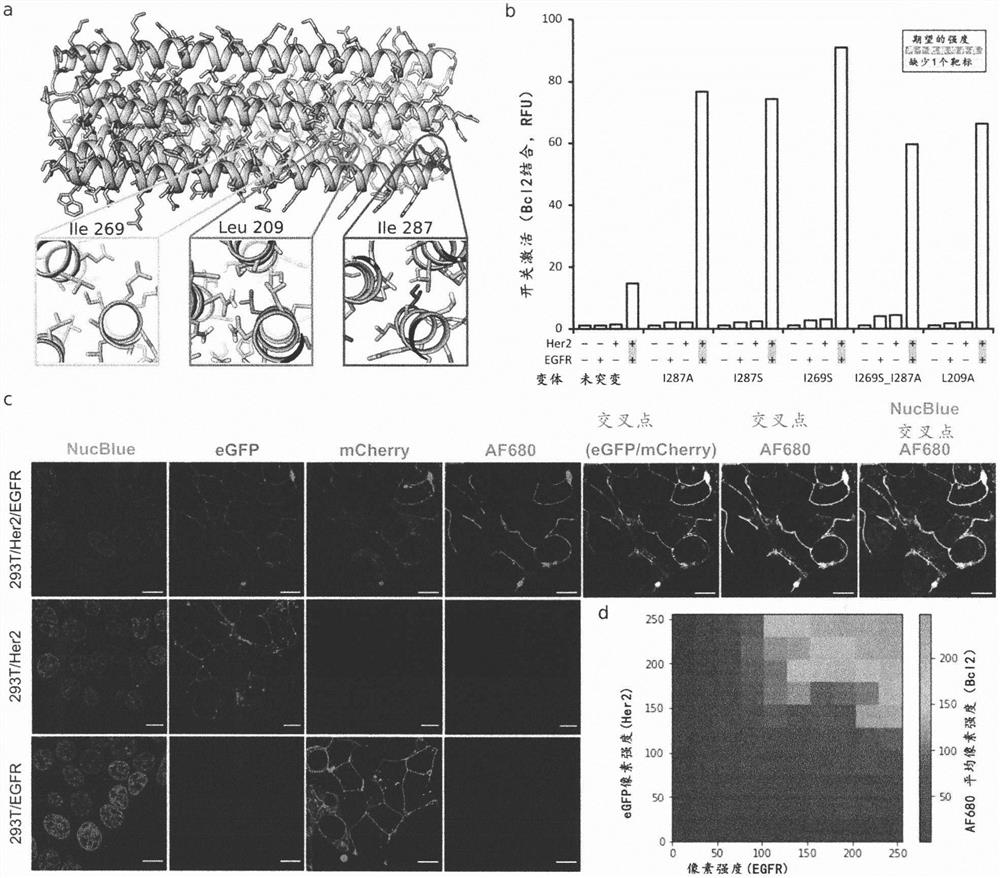
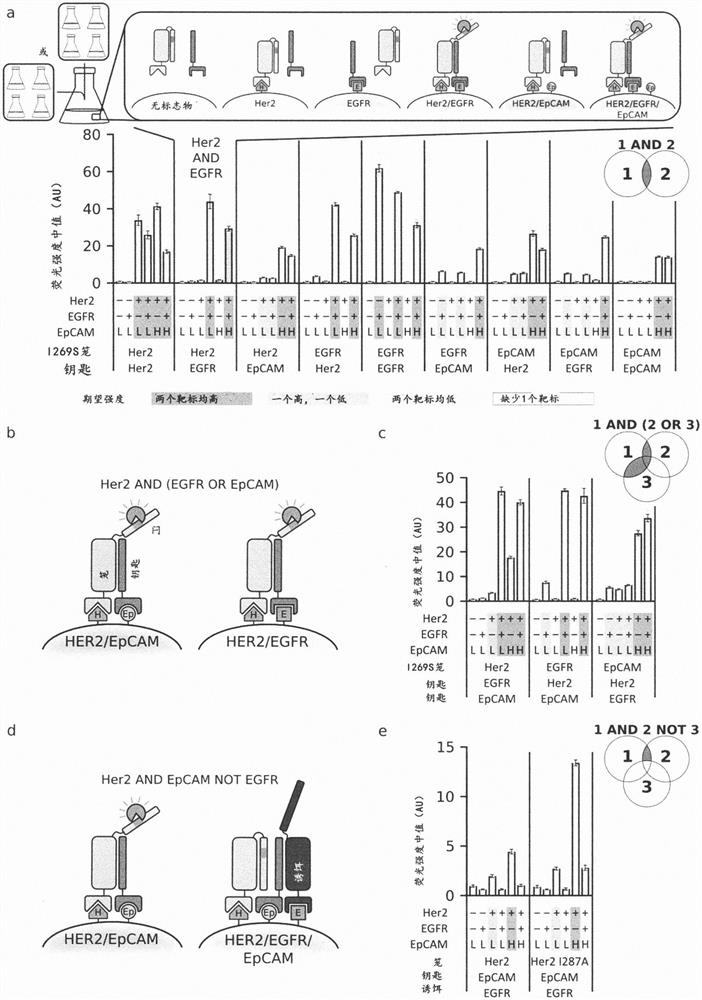
[0489] Natural biological systems integrate multiple protein-binding inputs through post-translational signaling cascades that are hard-coded into specialized functions; synthetic systems capable of integrating multiple binding inputs through conformational switching could be a general solution for predictively controlling multiple biological functions . We describe the computational design of proximity-activated de novo protein switches that perform AND, OR, and NOT Boolean logic operations, and combinations thereof, in response to precise combinations of protein-binding events. These switches are activated by a conformational change only if all logical conditions are met, and the high-resolution X-ray crystal structure confirms this design model. We demonstrate the utility of this system for hyperspecific targeting of mammalian cells, which are distinguished in complex cell populations only by their precise combination of surface markers. We implemented t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com