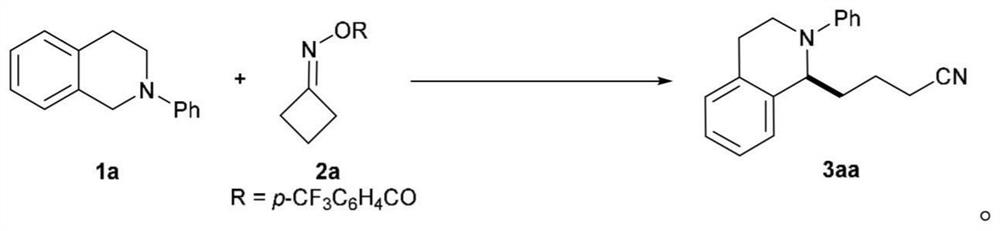Method for cross coupling of N-arylamine and cyclic ketoxime ester C (sp3)-C (sp3) through photooxidation reduction catalysis
A cross-coupling, arylamine technology, applied in the field of organic synthesis methodology, can solve problems such as chemical selectivity and poor atom economy, and achieve a wide range of reaction substrates, mild and simple catalytic reaction conditions, and reaction controllability. high effect
Active Publication Date: 2022-05-13
NANCHANG HANGKONG UNIVERSITY
View PDF6 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Typical methods for the alkylation of N-arylamines mainly rely on CDC (cross-dehydrocoupling) reactions, and although significant progress has been made in this area, there are still considerable limitations: (1) Most methods rely heavily on In activated alkyl C(sp 3 )-H bonds adjacent to heteroatoms (such as O, S, and N) or electron-withdrawing groups (such as nitriles, esters, ketones, and amides)
(2) usually require superstoichiometric oxidants and high temperatures, resulting in poor chemoselectivity and atom economy
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
[0059] The present invention will be further described in detail below in conjunction with specific embodiments. In the following, unless otherwise specified, the methods used are conventional methods in the art, and the reagents used can be purchased from conventional commercial channels and / or prepared according to known organic synthesis methods.
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
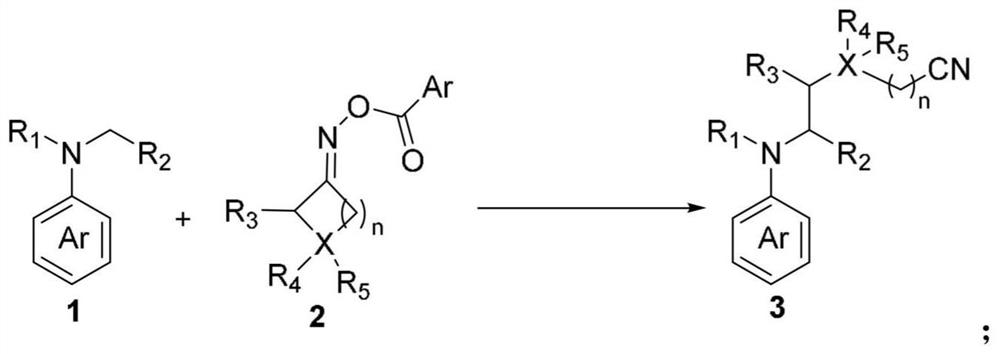
The invention discloses a method for cross coupling of N-arylamine and cyclic ketoxime ester C (sp3)-C (sp3) through photooxidation reduction catalysis. According to the method, cyclic ketoxime ester is taken as a cyanogen alkylation reagent, C (sp3)-C (sp3) cross-coupling reaction of N-arylamine and cyclic ketoxime ester is realized under the condition of no metal catalysis, various N-arylamine and cyclic ketoxime ester are converted into corresponding target compounds at good to excellent yield under mild conditions, and the method has a wide reaction substrate application range. The synthesis strategy can be applied to structural modification of d-aminocarbonyl compounds and oligopeptide compounds. According to the present invention, the catalytic reaction condition is mild and simple, the catalytic reaction can be smoothly performed at the room temperature by using the 3CzClIPN as the catalyst, the reaction controllability is high, the cost is low, the high regioselectivity can be obtained without using the metal catalyst, the alkali and / or the other ligand, and the method is suitable for the large-scale production;
Description
technical field [0001] This application belongs to the technical field of organic synthesis methodology, and specifically relates to a photoredox catalyzed N-arylamine and cyclic ketoxime ester C(sp 3 )-C(sp 3 ) cross-coupling method. Background technique [0002] Functional amine compounds are considered to be important structural units and widely exist in the structures of drug molecules and natural products. Due to their rich chemical and biological activities, the development of general synthetic strategies to prepare and / or modify this class of compounds has attracted increasing attention. Among the reported methods, the alkylation of N-arylamines is one of the most efficient ways to produce nitrogen-containing organic compounds, which introduces C(sp 3 ) part to change the spatial conformation and increase the lipophilicity and hydrophilicity of the drug compound. Typical methods for the alkylation of N-arylamines mainly rely on CDC (cross-dehydrocoupling) reaction...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07D217/14C07D217/18C07D217/16C07D295/185C07C253/00C07C253/34C07C255/28C07C255/42
CPCC07D217/14C07D217/18C07D217/16C07D295/185C07C253/00C07C253/34C07C255/28C07C255/42Y02P20/584
Inventor 李杨周生运李金恒张鼎
Owner NANCHANG HANGKONG UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com