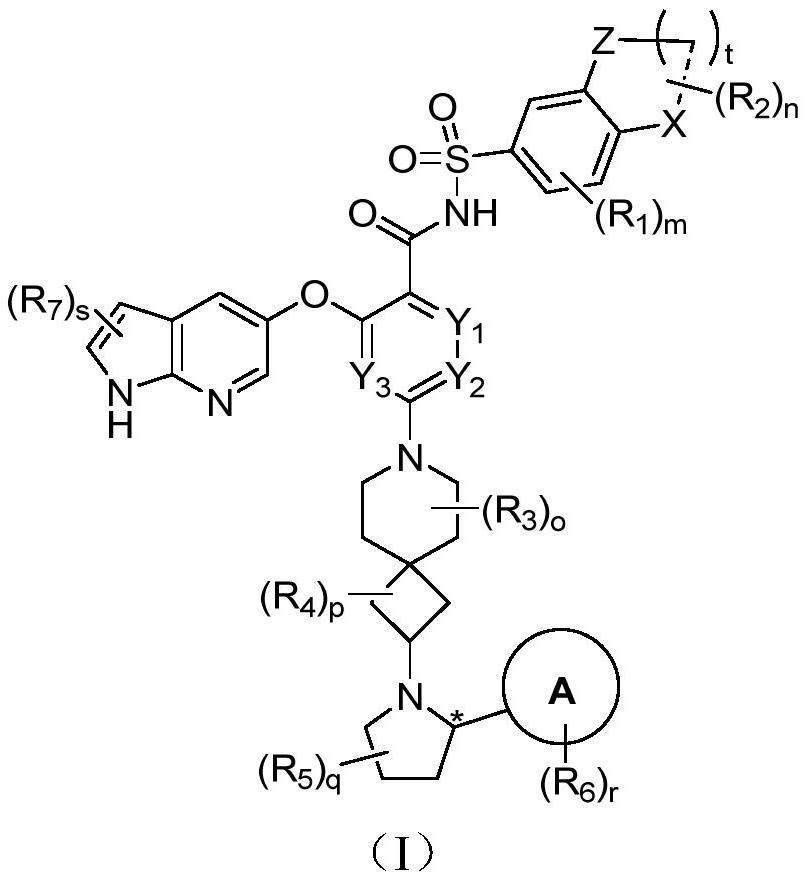
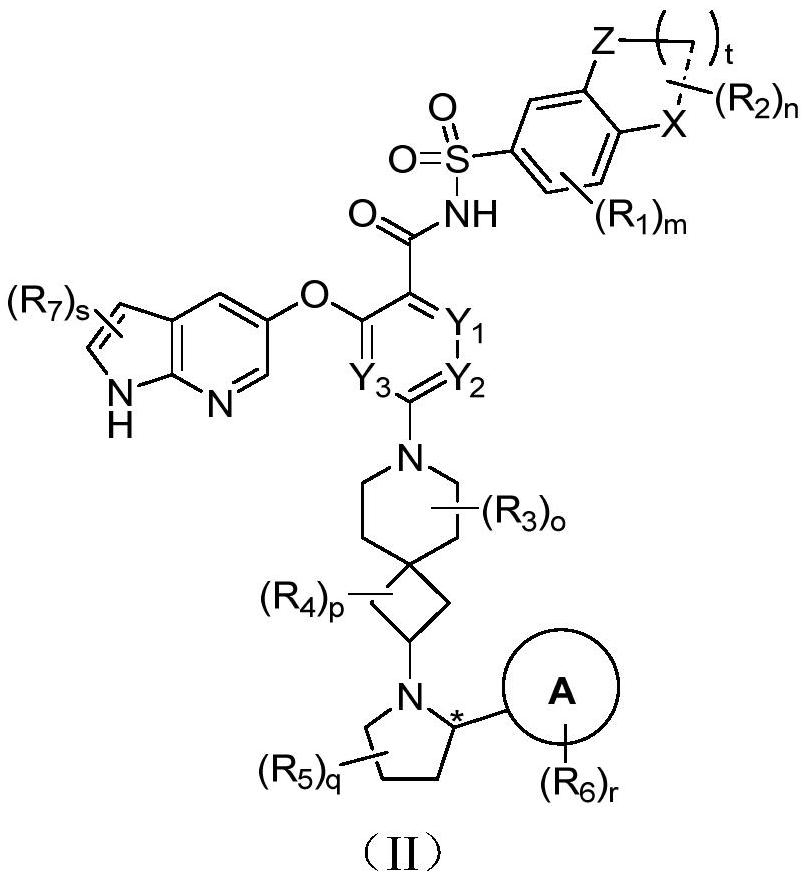
Bcl-2 protein apoptosis inducer and application
A general formula, stereoisomer technology, applied in the field of immunity and autoimmune diseases, can solve the problem of reducing the efficacy of BCL-2 inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
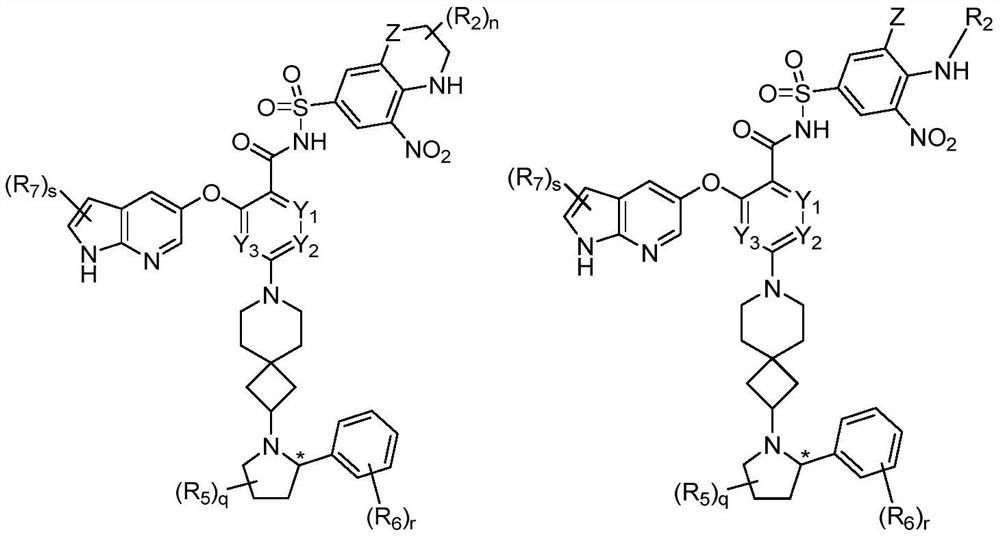
[0403] Example 1: (S)-2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-4-(2-(2-(2-isopropylphenyl) Pyrrolidin-1-yl)-7-azaspiro[3.5]nonan-7-yl)-N-((5-nitro-2H,4H-spiro[benzo[b][1,4] Oxazine-3,1'-cyclopropane]-7-yl)sulfonyl)benzamide (001)
[0404]
[0405] Step 1, Dissolve (S)-2-(2-bromophenyl)pyrrolidine-1-carboxylate tert-butyl ester (4.5 g) and isopropylboronic acid (3.1 g) in 15 mL of 1,4-epoxyhexa In the mixed solvent of ring and 3mL water, under nitrogen protection, add potassium carbonate (6g) and Pd(dffp) 2 Cl 2 (0.9g), heated to reflux overnight. TLC monitoring, after the reaction was completed, it was lowered to room temperature, ethyl acetate was added, washed with water and saturated brine respectively, the organic phase was separated and dried with anhydrous sodium sulfate, filtered, the filtrate was concentrated under reduced pressure, and the obtained crude product was purified by silica gel column chromatography to obtain the product (S)-tert-butyl 2-(2-isopropylph...
Embodiment 2
[0416] Example 2: (S)-3-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-5-(2-(2-(2-isopropylphenyl) Pyrrolidin-1-yl)-7-azaspiro[3.5]nonan-7-yl)-N-((5-nitro-2H,4H-spiro[benzo[b][1,4] Oxazine-3,1'-cyclopropane]-7-yl)sulfonyl)pyridinamide (002)
[0417]
[0418] The synthetic method of parameter embodiment 1, replace methyl 2,4-difluorobenzoate with methyl 3,5-difluoropyridine-2-carboxylate, can synthesize target compound (S)-3-((1H- Pyrrolo[2,3-b]pyridin-5-yl)oxy)-5-(2-(2-(2-isopropylphenyl)pyrrolidin-1-yl)-7-azaspiro[ 3.5] Nonan-7-yl)-N-((5-nitro-2H,4H-spiro[benzo[b][1,4]oxazine-3,1'-cyclopropane]-7- yl)sulfonyl)pyridinamide (90mg), LC-MS (ESI-MS): 833[M+H] + .
Embodiment 3
[0419] Example 3: (S)-2-((1H-pyrrolo[2,3-b]pyridin-5-yl)oxy)-3-fluoro-4-(2-(2-(2-isopropyl ylphenyl)pyrrolidin-1-yl)-7-azaspiro[3.5]nonan-7-yl)-N-((5-nitro-2H,4H-spiro[benzo[b][ 1,4]oxazine-3,1'-cyclopropane]-7-yl)sulfonyl)benzamide (003)
[0420]
[0421] The synthetic method of parameter embodiment 1, replace methyl 2,4-difluorobenzoate with methyl 2,3,4 trifluorobenzoate, can synthesize target compound (S)-2-((1H-pyrrolo [2,3-b]pyridin-5-yl)oxy)-3-fluoro-4-(2-(2-(2-isopropylphenyl)pyrrolidin-1-yl)-7-aza Spiro[3.5]nonan-7-yl)-N-((5-nitro-2H,4H-spiro[benzo[b][1,4]oxazine-3,1'-cyclopropane]- 7-yl)sulfonyl)benzamide (66mg), LC-MS (ESI-MS): 850[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com