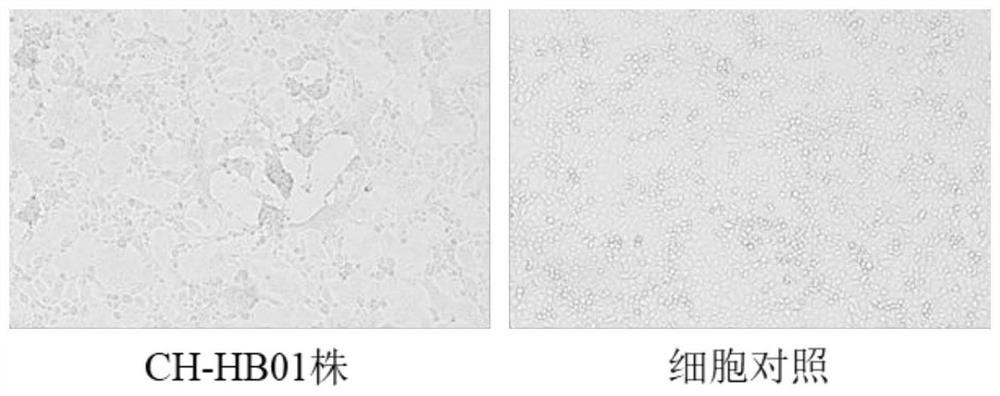
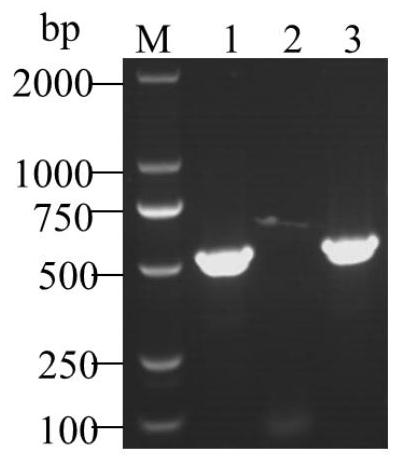
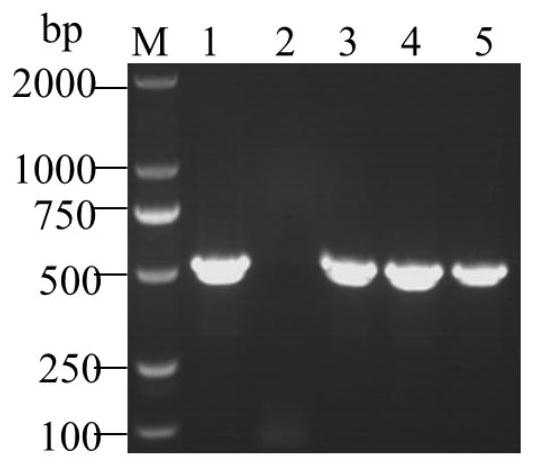
Porcine epidemic diarrhea and porcine rotavirus bivalent inactivated vaccine and preparation method thereof
A technology for porcine epidemic diarrhea and double inactivated vaccine, applied in the field of genetic engineering, can solve the problem of lack of immune effect, etc., and achieve the effects of good immunogenicity, high safety and high immune protection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1 Isolation, identification, cloning, purification and biological characteristics of porcine epidemic diarrhea virus
[0048] 1.1 Disease materials and cells
[0049] The disease materials were obtained from the small intestines of pigs with clinical symptoms of diarrhea in large-scale pig farms in my country, and Vero cells were kept and supplied by the laboratory of the R&D Center of Keqian Biological Co., Ltd. The porcine epidemic diarrhea virus used in this example is the porcine epidemic diarrhea virus 2aKQ01 strain recorded in the patent CN111041002B that has been deposited and the deposit number is: CCTCC NO: 202005.
[0050] 1.2 Isolation and identification of viruses
[0051] Use specific primers to amplify the PEDV M gene. The primer sequences are:
[0052] Upstream primer PEDV-MS: 5'-ATGTCTAACGGTTCTATTCCCGT-3'
[0053] Downstream primer PEDV-MA: 5'-CTTGGCGACTGTGACGAAAT-3'
[0054] The size of the amplified fragment was 519bp, and the primers were ...
Embodiment 2
[0094] Example 2 Isolation, identification, cloning, purification and biological characteristics of porcine rotavirus
[0095] 1.1 Disease materials and cells
[0096] The disease materials were obtained from the small intestines of pigs with clinical symptoms of diarrhea in large-scale pig farms in my country, and the MA104 cells were kept and supplied by the laboratory of the R&D Center of Keqian Biological Co., Ltd. Suspension PK-15 cells were kept and supplied by Wuhan Keqian Biological Co., Ltd. In the present invention, the medium for suspension culture of PK-15 cells (PK serum-free medium) was purchased from Zhejiang Yishengke Biotechnology Co., Ltd., item number ZJ02F1170.
[0097] 1.2 Isolation and identification of viruses
[0098] Use specific primers to amplify the PoRV VP6 gene. The primer sequences are:
[0099] Upstream primer PoRV-jdS: 5'-ATTTGATGGGAACTATGTGG-3'
[0100] Downstream primer PoRV-jdA: 5'-GGTGGACGAACTAATTGAAACG-3'
[0101] The size of the ampl...
Embodiment 3
[0188] Example 3 Preparation and inspection of porcine epidemic diarrhea and porcine rotavirus dual inactivated vaccine
[0189] Another object of the present invention is to provide a preparation method of porcine epidemic diarrhea and porcine rotavirus dual inactivated vaccine, comprising the following steps:
[0190] 1 Preparation of porcine epidemic diarrhea virus (porcine epidemic diarrhea virus 2A KQ01 strain) virus liquid
[0191] 1.1 Cell expansion culture in bioreactors
[0192] When the cells in the seed tank are cultured for 72h or 96h and the cell viability is above 95%, the cells are transferred to the bioreactor. Before inoculating cells, the dissolved oxygen (DO) electrode, pH electrode, and temperature electrode should be calibrated, and the tank should be autoclaved. Pump 20% of the total volume of the bioreactor with ST serum-free medium to 1 x 10 6 Cells were transferred to a bioreactor at a cell density of cells / ml, and the optimal culture conditions wer...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com