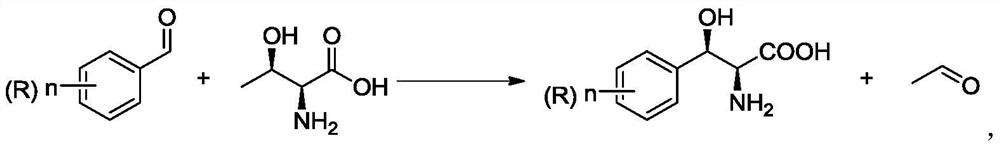
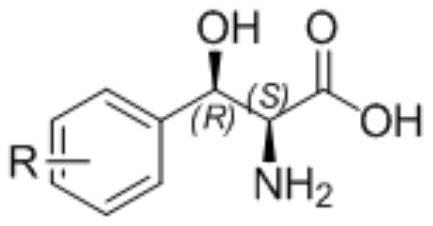
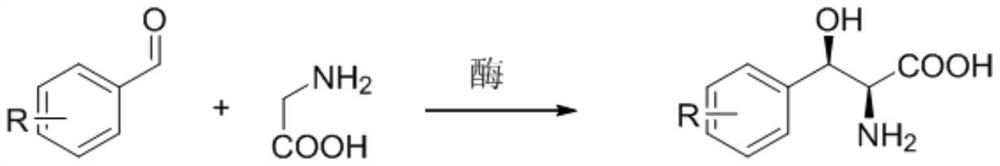
Immobilized modified threonine transaldolase and application thereof
A technology for immobilizing enzymes and threonine, applied in the direction of immobilizing enzymes, transferases, oxidoreductases, etc., can solve the problems of high cost and large amount of enzymes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Preparation of immobilized enzyme catalyst
[0037] Unless otherwise specified, the experimental methods used in the present invention are all conventional methods.
[0038] i) Reagents and instruments:
[0039] p-Methylsulfonylbenzaldehyde was purchased from Aladdin, item number M185093, purity 98%;
[0040] L-threonine was purchased from McLean, product number C10393311, analytically pure;
[0041] Pyridoxal phosphate was purchased from Aladdin, item number P136795, purity ≥98%;
[0042] Magnesium chloride was purchased from Aladdin, article number A2006034, analytically pure;
[0043] Oxidized nicotinamide adenine dinucleotide (NAD) was purchased from Aladdin, catalog number N196974, with a purity of 95%.
[0044] LX-1000NH was purchased from Lanxiao Technology.
[0045] ii) Vector and strain: the used expression vector was pET-30a(+), the plasmid was purchased from Novagen Company, and the used host cell was Escherichia coli BL21 (DE3), purchased fro...
Embodiment 2
[0060] Example 2: Preparation of immobilized enzyme (2S,3R)-p-methylsulfonylphenylserine
[0061] p-methylsulfonylbenzaldehyde 50g / L, L-threonine 42g / L, magnesium chloride 1g / L, pyridoxal phosphate 0.15g / L, co-substrate (see Table 2 for specific amounts), 100mM phosphate buffer (pH7 0) be heated to 30 ℃ of magnetic stirring uniformly, add the immobilized enzyme prepared in Example 1, start stirring reaction, react 24 hours sampling HPLC detection conversion rate (for the first time), apply mechanically after 30 times and detect conversion rate (results are shown in Table 3) .
[0062]
[0063] table 3
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com