Preparation method of N, N-bis (2-chloroethyl)-4-nitrophenyl alanine methyl ester
A technology of nitrophenylalanine methyl ester and chloroethyl, which is applied in the field of preparation of N,N-di-4-nitrophenylalanine methyl ester, can solve problems such as no preparation method, and achieves The effect of the simple operation of the reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
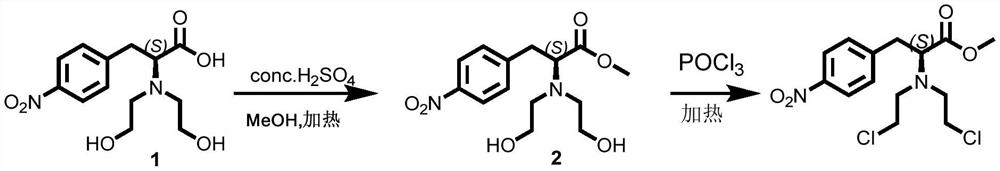
[0024] A preparation method of N,N-bis(2-chloroethyl)-4-nitrophenylalanine methyl ester, the preparation method comprising the steps of:
[0025] Compound 1 (N,N-bis(2-hydroxyethyl)-4-nitrophenylalanine) (6.0g, 0.02mol) was added to 200mL of methanol, and slowly added 0.5 mL of 18mol / L concentrated sulfuric acid, then warmed up to 60°C and stirred for 24 hours. After the reaction was detected by TLC, it was concentrated to dryness by distillation under reduced pressure, and purified by column (200-300 mesh silica gel), using dichloromethane: Methanol = 40:1 solvent for isocratic elution, the product part was collected, concentrated to dryness under reduced pressure to obtain a yellow solid, that is, the compound 2(N,N-bis(2-hydroxyethyl)-4-nitro Phenylalanine methyl ester) crude product 5.6g, directly used in the next step of chlorination without further purification.
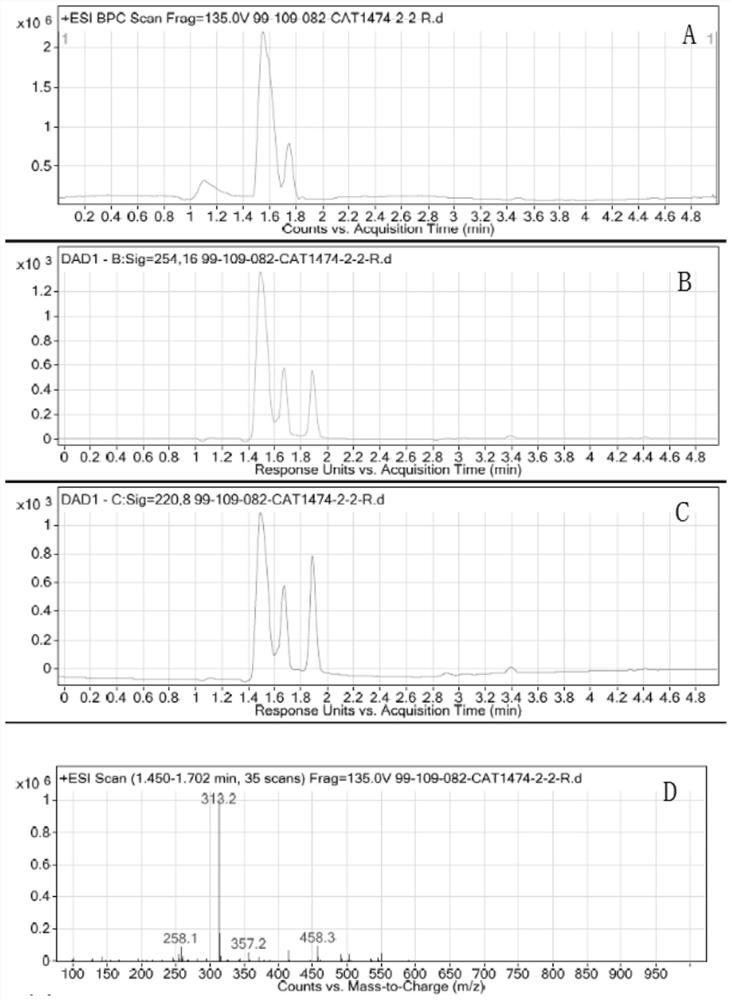
[0026] The LCMS spectrum of compound 2 is as follows figure 1 Shown, in the figure A: ion flow chart; B: t...
Embodiment 2
[0030] A preparation method of N,N-bis(2-chloroethyl)-4-nitrophenylalanine methyl ester, the preparation method comprising the steps of:
[0031] Compound 1 (N,N-bis(2-hydroxyethyl)-4-nitrophenylalanine) (6.0g, 0.02mol) was added to 10mL of methanol, and 5.5 mL of 18mol / L concentrated sulfuric acid, then heated up to 80°C and stirred for 4 hours. After the reaction was detected by TLC, it was concentrated to dryness by distillation under reduced pressure, and then purified by column (200-300 mesh silica gel), using dichloromethane: Methanol = 40:1 solvent for isocratic elution, the product part was collected, concentrated to dryness under reduced pressure to obtain a yellow solid, that is, the compound 2(N,N-bis(2-hydroxyethyl)-4-nitro Phenylalanine methyl ester) crude product 5.2g, directly used in the next step of chlorination without further purification.
[0032] Compound 2 (N,N-bis(2-hydroxyethyl)-4-nitrophenylalanine methyl ester) (5.2g, 0.017mol) was slowly added in ba...
Embodiment 3
[0034] Compound 1 (N,N-bis(2-hydroxyethyl)-4-nitrophenylalanine) (6.0g, 0.02mol) was added to 100mL of methanol, and 2.5 mL of 18mol / L concentrated sulfuric acid, then heated up to 80°C and stirred for 4 hours. After the reaction was detected by TLC, it was concentrated to dryness by distillation under reduced pressure, and then purified by column (200-300 mesh silica gel), using dichloromethane: Methanol = 40:1 solvent for isocratic elution, the product part was collected, concentrated to dryness under reduced pressure to obtain a yellow solid, that is, the compound 2(N,N-bis(2-hydroxyethyl)-4-nitro Phenylalanine methyl ester) crude product 5.5g, directly used in the next step of chlorination without further purification.
[0035] Compound 2 (N,N-bis(2-hydroxyethyl)-4-nitrophenylalanine methyl ester) (5.5g, 0.018mol) was slowly added in batches to phosphorus oxychloride (85mL, 0.87mol ), then heated up to 95°C and stirred for 12 hours. After the reaction was detected by TLC,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com