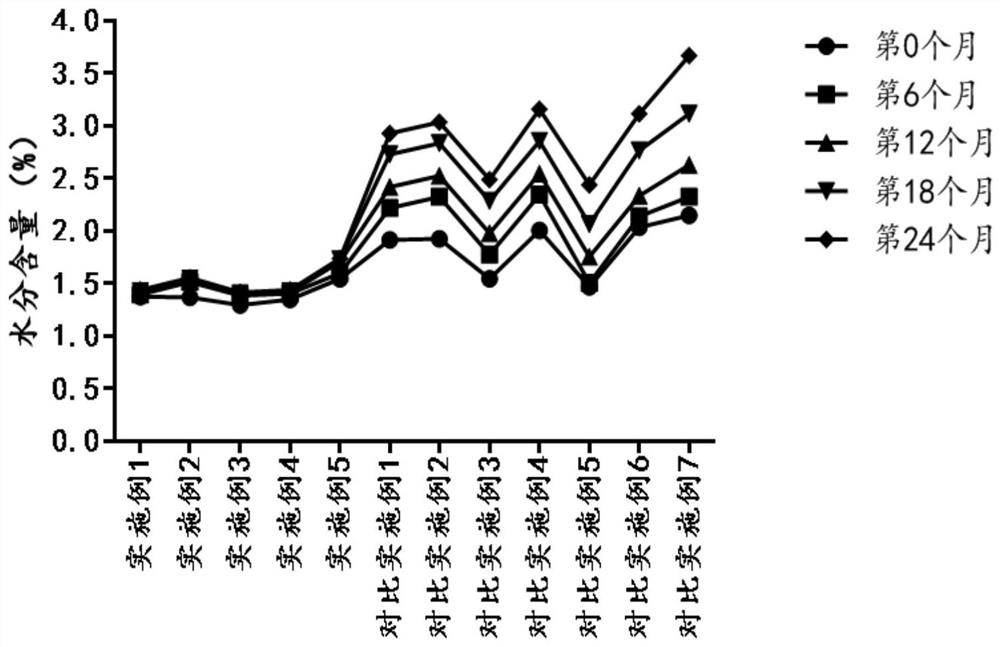
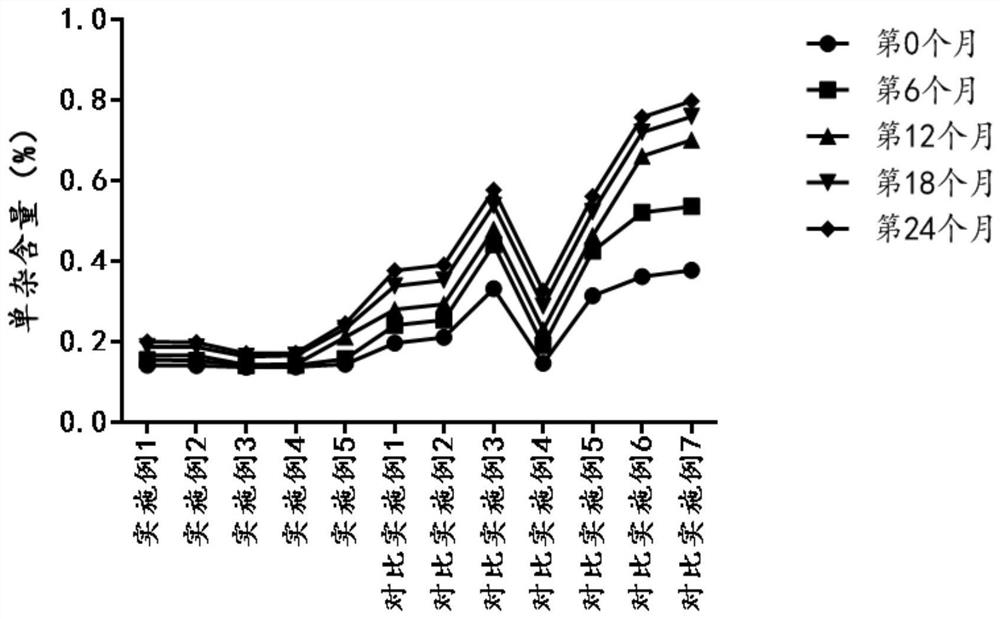
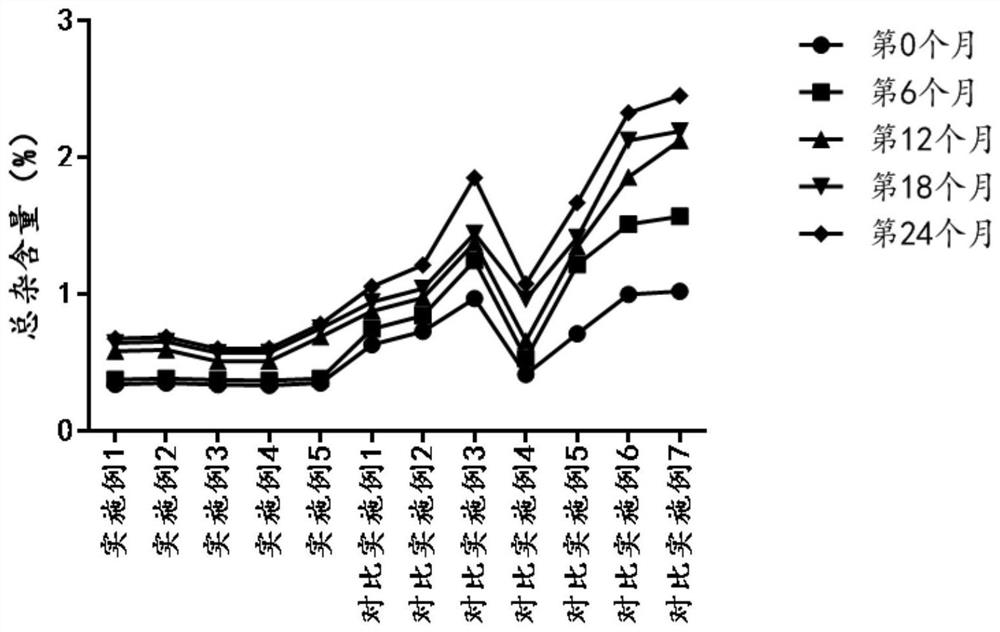
Freeze-dried powder containing ritodrine hydrochloride as well as application and preparation method thereof
A technology of ritodrine hydrochloride and freeze-dried powder, which is applied in the directions of medical preparations containing active ingredients, medical preparations without active ingredients, and freeze-dried transportation, so as to achieve the advantages of expanding production, facilitating transportation and storage, and reducing transportation costs. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: a kind of ritodrine hydrochloride freeze-dried powder, described component content and preparation technology are as follows:
[0034]
[0035] Preparation Process:
[0036] (1) take by weighing sorbitol, add an appropriate amount of water for injection, stir and dissolve, add tert-butanol and ritodrine hydrochloride successively, stir to dissolve the main drug completely;
[0037] (2) Add activated carbon, stir for more than 10 minutes, decarbonize, and add water for injection to the full amount;
[0038] (3) using citric acid to adjust the pH value to 4.5, and filling with nitrogen for protection;
[0039] (4) filling the medicinal liquid in the ampoule bottle and freeze-drying; the freeze-drying curve is:
[0040] S1 pre-freezing treatment: cool down to -40±5℃, keep for 2-3 hours;
[0041] S2 heating stage: raise the temperature to -20±5°C, and keep the temperature for 2 to 4 hours;
[0042] S3 cooling stage: cool down to -35±5℃, keep warm for 2-...
Embodiment 2
[0046] Embodiment 2: a kind of ritodrine hydrochloride freeze-dried powder, described component content and preparation technology are as follows:
[0047]
[0048] The preparation process is the same as:
[0049] (1) take by weighing sorbitol, add an appropriate amount of water for injection, stir and dissolve, add tert-butanol and ritodrine hydrochloride successively, stir to dissolve the main drug completely;
[0050] (2) Add activated carbon, stir for more than 10 minutes, decarbonize, and add water for injection to the full amount;
[0051] (3) use citric acid to adjust pH value to be 6.0, and fill with nitrogen protection;
[0052] (4) filling the medicinal liquid in the ampoule bottle and freeze-drying; the freeze-drying curve is:
[0053] S1 pre-freezing treatment: cool down to -40±5℃, keep for 2-3 hours;
[0054] S2 heating stage: raise the temperature to -20±5°C, and keep the temperature for 2 to 4 hours;
[0055] S3 cooling stage: cool down to -35±5℃, keep wa...
Embodiment 3
[0059] Embodiment 3: a kind of ritodrine hydrochloride freeze-dried powder, described component content and preparation technology are as follows:
[0060]
[0061]
[0062] Preparation Process:
[0063] (1) take by weighing sorbitol, add an appropriate amount of water for injection, stir and dissolve, add tert-butanol and ritodrine hydrochloride successively, stir to dissolve the main drug completely;
[0064] (2) Add activated carbon, stir for more than 10 minutes, decarbonize, and add water for injection to the full amount;
[0065] (3) use glycine to adjust the pH value to 5.0, and fill with nitrogen for protection;
[0066] (4) filling the medicinal liquid in the ampoule bottle and freeze-drying; the freeze-drying curve is:
[0067] S1 pre-freezing treatment: cool down to -40±5℃, keep for 2-3 hours;
[0068] S2 heating stage: raise the temperature to -20±5°C, and keep the temperature for 2 to 4 hours;
[0069] S3 cooling stage: cool down to -35±5℃, keep warm for 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com