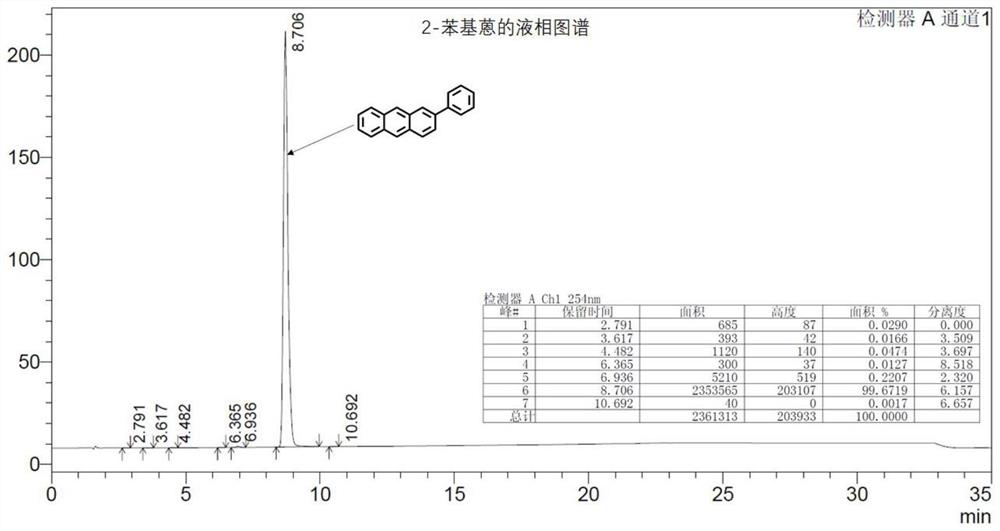
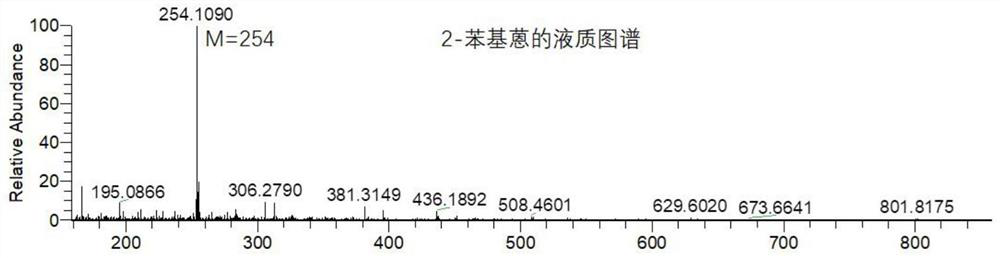
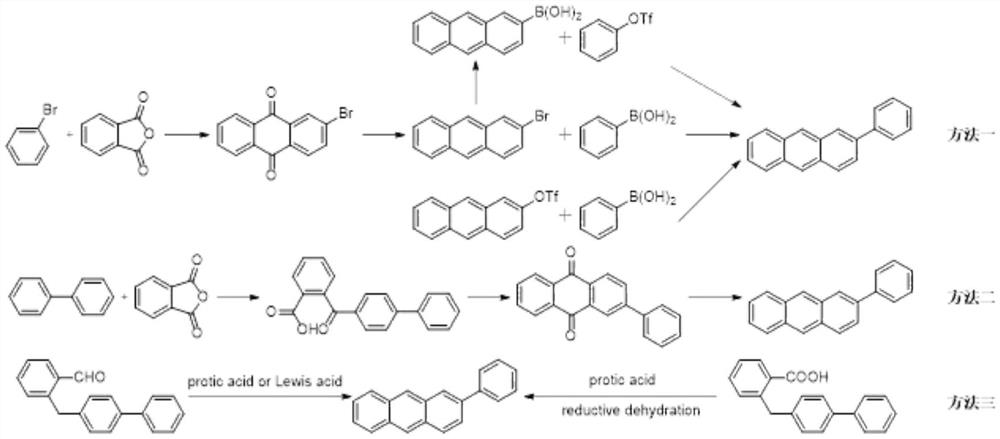
Synthesis method of 2-phenyl anthracene
A synthesis method and technology of phenylanthracene, which is applied in the field of synthesis of 2-phenylanthracene, can solve the problems of many impurities, difficulty in obtaining raw materials, and high production cost, and achieve the effects of high yield, reduced production cost, and low environmental hazards
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] This embodiment provides an industrialized production method of 2-phenylanthracene.
[0025] The synthesis process is as follows:
[0026] To a 1L three-necked round-bottomed flask, 286 mL of tetrahydrofuran, 28.60 g (0.1 mol) of 2,3-dibromonaphthalene were successively added, the temperature was lowered to -80°C to -70°C with stirring, and 60 mL of n-butyllithium (2mol / L, 0.12mol), stir for 0.5h, add 10.97g (0.15mol) of N,N-dimethylformamide, add dropwise, keep at -85~-70°C and stir for 3h, naturally heat up to -20°C, add hydrochloric acid to acidify to pH =7, naturally rise to room temperature, concentrate under reduced pressure until the inner temperature is equal to 45°C, add 286 mL of toluene to dilute, separate the phases, concentrate the organic phase at normal pressure to the remaining 30 mL of the system, add 143 mL of n-heptane, put it in the refrigerator for 3 hours of freezing and crystallization, Filter and bake to constant weight to obtain 20.30 g of off-...
Embodiment 2
[0039] This embodiment provides an industrialized production method of 2-phenylanthracene.
[0040] The synthesis process is as follows:
[0041] To a 1L three-necked round-bottomed flask, 286 mL of tetrahydrofuran and 28.60 g (0.1 mol) of 2,3-dibromonaphthalene were successively added, the temperature was lowered to -80°C to -70°C with stirring, and 90 mL of n-butyllithium (2mol / L, 0.18mol), stir for 0.5h, add 18.26g (0.25mol) of N,N-dimethylformamide, add dropwise, keep at -85~-70°C, stir for 3h, naturally heat up to -20°C, add hydrochloric acid to acidify to pH =7, naturally rise to room temperature, concentrate under reduced pressure until the inner temperature is equal to 45°C, add 286 mL of toluene to dilute, separate the phases, concentrate the organic phase at normal pressure to the remaining 30 mL of the system, add 143 mL of n-heptane, put it in the refrigerator for 3 hours of freezing and crystallization, Filter and bake to constant weight to obtain 19.88 g of an o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com