Feline calicivirus vaccine
A feline calicivirus, virus technology, applied in the direction of viruses, vaccines, viral peptides, etc., can solve the problems of lack of extensive cross-protection, no development, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0117] Neutralization indices for several FCV strains were determined using hyperimmune sera raised in cats against strains FCV F9 and Kalem Crouch. The experiment was performed as described in Section 8 below. The data are shown in Table 1. It becomes clear from the table that sera raised against Kalem Crouch have broad cross-protection against many other FCV strains. For sera against Kalem Crouch, significant Logs were seen against 16 out of 31 FCV strains 10 Decrease (ie > 1.5). Table 1 also shows that the cross-protection of the commonly used F9 strain is much lower. For 3 of the 22 FCV strains, sera raised against F9 showed significant Log 10 decrease (ie >1.5). It should be noted that the 2 FCV strains 3809, 6420, CV-21 that were neutralized or at least significantly reduced by F9 serum were F9-like viruses. Thus, not only does Kalem Crouch cross-protection for much more FCV strains than F9, but it also provides cross-protection for non-F9 strains.
[0118] Table ...
Embodiment 2
[0121] Construction of hybrid FCV-clones.
[0122] 1. cell culture
[0123] All cell lines were grown at 37°C, 5% CO 2 maintained in tissue culture flasks.
[0124] Cradell-Rees Feline Kidney (CrFK) cells were cultured in cells supplemented with 5% fetal bovine serum, 0.15% sodium bicarbonate, 2 mM L-glutamine, 100 U / ml penicillin, 10 μg / ml streptomycin, and 2 μg / ml amphotericin B grown in medium M6B8.
[0125] BsRT7 cells were maintained in medium DMEM supplemented with 5% fetal bovine serum, 2 mM L-glutamine, 1 mM sodium pyruvate, and 1 mg / ml Geneticin (G418). Geneticin was removed at cell seeding prior to transfection.
[0126] 2. virus isolation
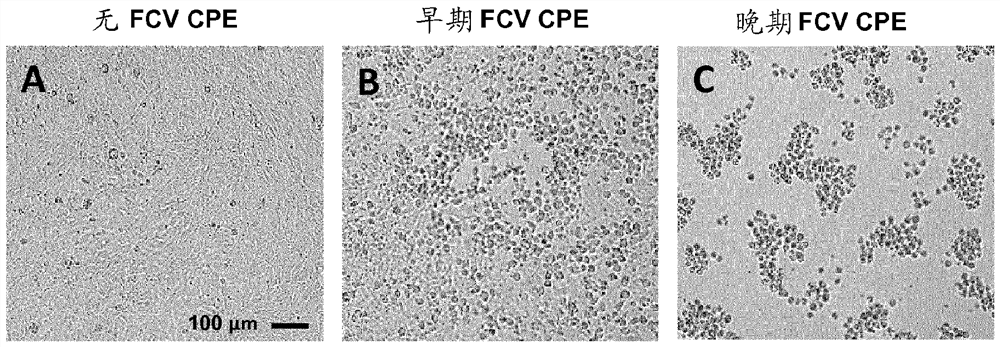
[0127] Oropharyngeal / nasal swabs were collected from cats and transported in medium M6B8. The swab was briefly vortexed and the virus suspension was inoculated onto confluent CrFK cells and incubated at 37°C and 5% CO. 2 Incubate until FCV-specific CPE is observed. Infected flasks were freeze-thawed to lyse cells, cla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com