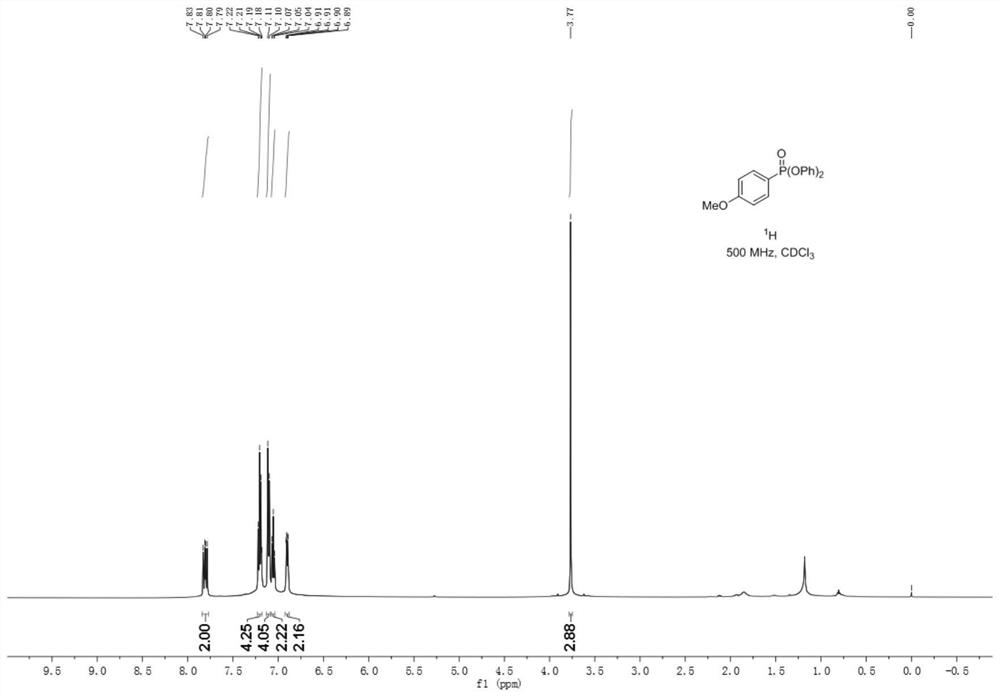
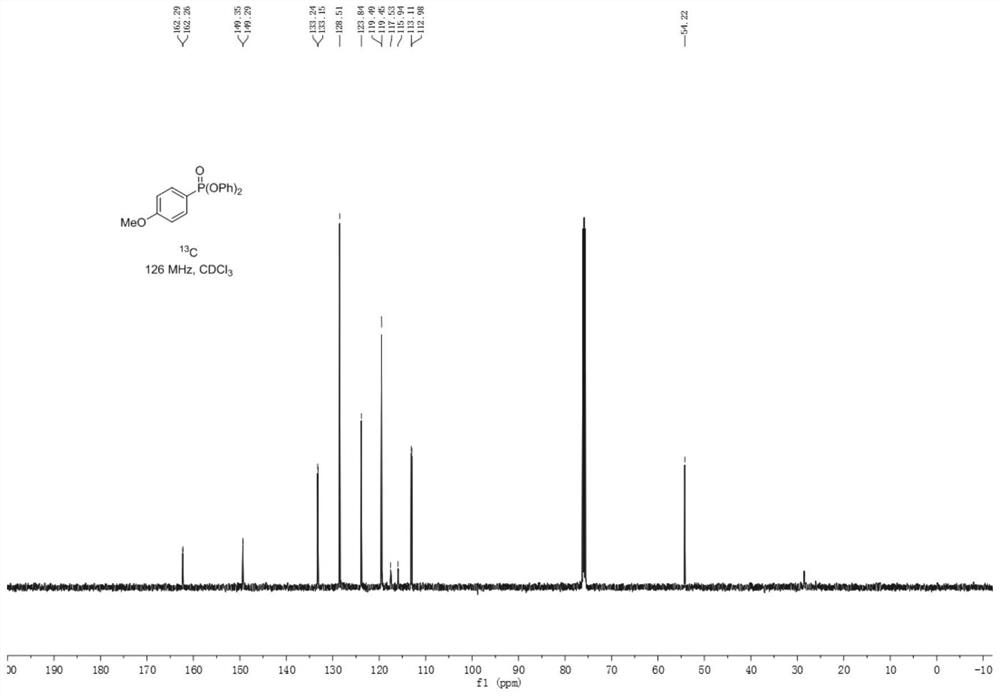
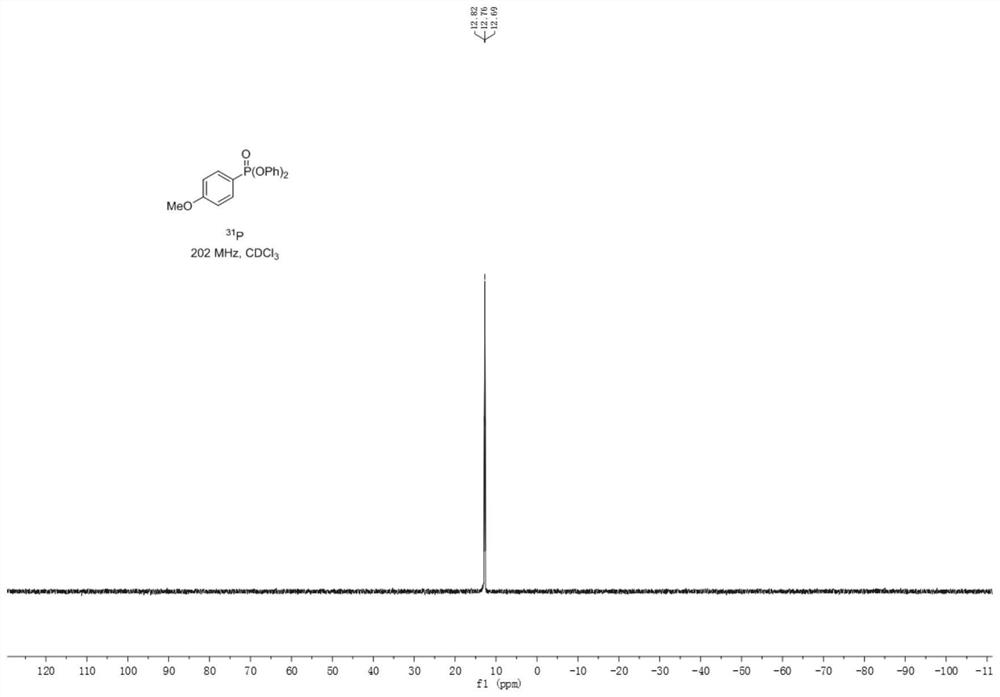
Synthetic method of aryl phosphate
A technology of aryl phosphate ester and synthesis method, applied in electrolytic components, electrolytic organic production, electrolytic process, etc., to achieve high selectivity, mild reaction conditions, and avoid the effects of derivatization reactions
Pending Publication Date: 2022-06-10
重庆化工职业学院
View PDF5 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
No photocatalyst is used in the reaction system and the reaction conditions are mild, but there is a problem that the reaction time is too long
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention provides a synthesis method of aryl phosphate ester, which comprises the following steps: taking aryl azo sulfone as a raw material, and reacting with triphenyl phosphite under electrochemical conditions to obtain a corresponding aryl phosphate ester compound. The method has the advantages of cheap and easily available raw materials, simple operation, mild reaction conditions, greenness, high yield and greatly shortened reaction time. The method provided by the invention utilizes electrons as reactants to realize selective oxidation or reduction conversion, avoids the use of stoichiometric oxidants, metal catalysts and alkalis, and meets the requirements of green chemical development. The reaction has high selectivity, so that derivatization reactions such as protection / deprotection are avoided, and the method is suitable for industrial production.
Description
technical field [0001] The invention relates to a novel synthesis method of aryl phosphate compounds, belonging to the technical field of electrochemical organic synthesis. Background technique [0002] As an important class of organophosphorus compounds, aryl phosphonates are widely used in the fields of medicinal chemistry, material science and organic synthesis, which makes the synthesis of such compounds of great significance. Phosphorous moieties act as ligands to coordinate with transition metals or bind to biological receptors, thereby modulating physiological processes or material functions. Therefore, the development of efficient and mild phosphonylation reactions has been the subject of extensive synthetic research. The field has long been dominated by transition metal catalysis, but reports on the use of visible-light photoredox catalysis and electrocatalysis as a mild and efficient strategy to access aryl phosphonates have attracted increasing attention in recen...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C25B3/07C25B3/23
CPCC25B3/07C25B3/23
Inventor 王荣康马昱博揭芳芳
Owner 重庆化工职业学院
Who we serve
- R&D Engineer
- R&D Manager
- IP Professional
Why Patsnap Eureka
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com