A kind of monoclonal antibody e44 against Rift Valley fever virus and its application
A monoclonal antibody, Rift Valley fever technology, applied in the field of microbiology and immunology, to achieve good stability, good neutralization protection effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Screening and preparation of anti-RVF monoclonal antibodies
[0038] 1.1 Packaging of human recombinant adenovirus type 5 (HAdV5-GnGcopt) expressing Rift Valley fever virus GnGc protein
[0039] The recombinant GnGc protein-encoding gene (GenBank: DQ380208.1) was cloned into the expression vector pDC316, and then co-transfected with the backbone plasmid (pBHGlox_E1, Cre) of the AdMax adenovirus system into HEK293 cells. After inoculation into 293F cells for propagation, the virus titer was measured and stored at -80°C for use (see CN105483140A for the construction method).
[0040] 1.2 Immunization of rhesus monkeys
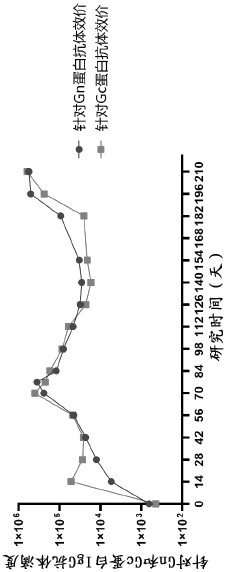
[0041] First, 2 mL of rhesus monkey blood was collected intravenously before immunization and the serum was separated as a negative serum control. At the same time, 1×10 8Rhesus monkeys were immunized with IFUs of HAdV5-GnGcopt by intramuscular injection. Rhesus monkeys were immunized again with the same dose of HAdV5-GnGcopt on the 28th day a...
Embodiment 2
[0151] Example 2. Pharmacodynamic studies
[0152] 2.1 Determination of Neutralizing Antibody Affinity
[0153] Protein A chip was used to detect the affinity of E44 and Gn protein by Biacore T200 instrument. The antibody was first diluted to 0.5 μg / mL, and the antibody was captured by the Protein A chip at a flow rate of 10 μL / min. The Gn protein was then diluted to 320nM, 160nM, 80nM, 40nM and 20nM and passed through the E44-captured Protein A chip at a flow rate of 30μL / min and bound for 120s to determine the binding rate (K a ), and then dissociated for 600 s to determine the dissociation rate (K d ). Finally, the affinity of the antibody to be tested is calculated by the ratio of Ka and Kd (K D ). Finally, the affinity between E44 and Gn protein was measured to be 2..58nM. The results are shown in Figure 13 As shown in Table 16, it can be seen from the graph that the binding speed of E44 and Gn protein is faster and the dissociation speed is slower, so the affinity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com