Development and application of immunomodulator
A sequence and antibody technology, applied in the field of binding programmed death ligand 1 antibody
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0180] Example 1. PD-L1 humanized antibody
[0181] The PD-L1 antibody is derived from the mouse hybridoma clone PDL1-3F2 obtained from immunized animals, and its antibody variable region sequence is as follows:
[0182] The amino acid sequence of the light chain variable region of PDL1-3F2 is shown in SEQ ID NO.1, its CDR1, CDR2 and CDR3 are shown in SEQ ID NO.2, 3, and 4, respectively, and its encoding nucleic acid is shown in SEQ ID NO.5 Show.
[0183] CDR1CDR2 RASQSISNNLH WYQQKSHESPRLLIK YASQSIS GIPSRFSGSGSGT--FR3------------->CDR3
[0184] DFTLSINSVETEDFGMYFC QQSKSWPFTF GSGTRLEIK
[0185]Nucleic acid sequence
[0186] GATATTGTGCTAGCTCAGTCTCCAGCCACCCTGTCTGTGACTcCAGGAGATAGCGTCAGTCTTTCCTGCAGGGCCAGCCAAAGTATTAGCAACAACCTACACTGGTATCAACAAAAATCACATGAGTCTCCAAGGCTTCTCATCAAGTATGCTTCCCAGTCCATCTCTGGGATCCCCTCTAGGTTCAGTGGCAGTGGATCAGGGACAGATTTCACTCTCAGTATCAACAGTGTGGAGACTGAAGATTTTGGAATGTATTTCTGTCAACAGAGTAAAAGCTGGCCATTCACGTTCGGCTCGGGGACAAGGTTGGAAATAAAA
[0187] The amino acid sequen...
Embodiment 2
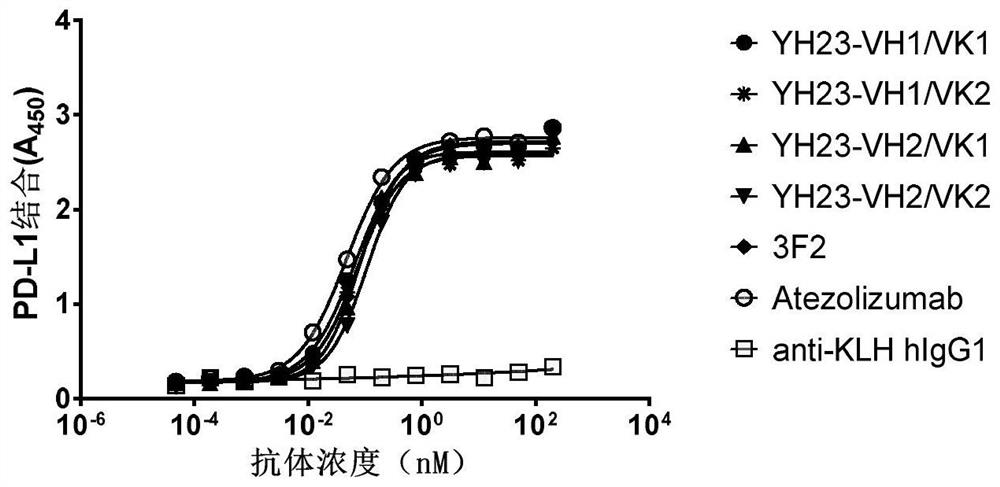
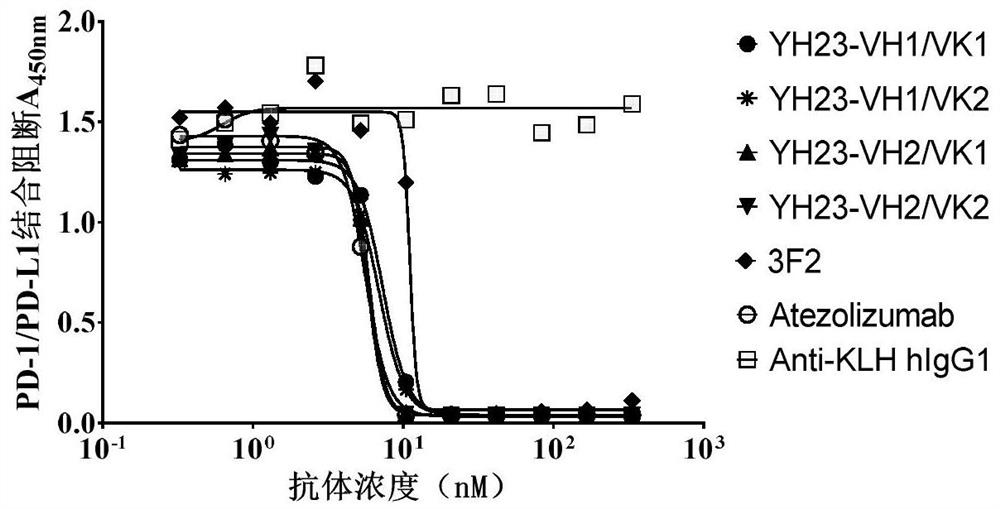
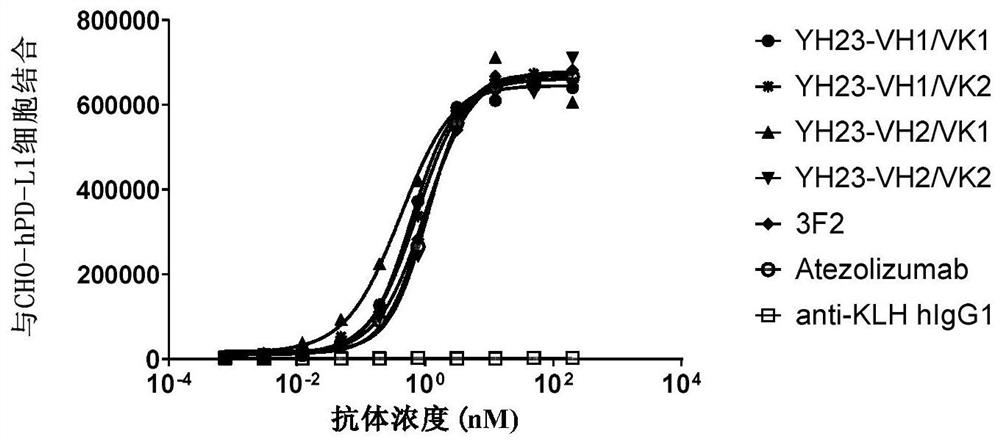
[0218] Example 2. Binding and blocking activity of PD-L1 humanized antibody
[0219] 1. ELISA detection of humanized antibody binding to human PD-L1
[0220] 1 μg / ml human PD-L1 protein (Sinobiological #10084-H08H) was coated on a 96-well microtiter plate at 4°C overnight. After blocking with 2% milk, 100 μl of the recombinantly expressed PD-L1 humanized antibody prepared in Example 1 was added to each well, and incubated at room temperature for 1 hour; after washing three times with PBST and PBS, 100 μl of HRP-labeled antibody was added to each well. Human IgG F(ab)' 2 Secondary antibody, incubated at room temperature for 60 minutes. After washing three times with PBST and PBS, 100 μl of TMB substrate was added to each well for 10 minutes at 37° C. After color development, the reaction was terminated with 50 μl of 2M sulfuric acid solution per well, and the absorbance was read at a wavelength of 450 nm. The control antibody atezolizumab was synthesized according to the pub...
Embodiment 3
[0234] Example 3. Affinity determination of humanized antibody and PD-L1
[0235] 10 μg / ml of human PD-L1 recombinant protein was coupled to a CM5 chip (GEhealthcare) by amino coupling reagent, the flow rate was 10 μl / min, and the amount of antigen coupling was controlled by Biacore T200 to about 200RU. After the chip was rinsed with buffer until the baseline was stable, the PD-L1 antibody prepared in Example 1 with gradient dilution (from 10 μg / ml, 2-fold dilution for 7 gradients) was flowed through the chip at a flow rate of 30 μl / min, and the binding was set. Time 350 seconds, dissociation time 600 seconds. A 1:1 binding model was fitted with Biacore T200 evaluation software to obtain kinetic constants (Table 4).
[0236] Table 4. Affinity of PD-L1 humanized antibody and PD-L1 recombinant protein
[0237]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com