Application of DOR agonist in preparation of medicine for resisting renal fibrosis
A kidney fibrosis and agonist technology, applied in the field of medicine, can solve the problems of expensive medical expenses, high morbidity and poor prognosis, and achieve the effect of reducing fibrotic lesions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
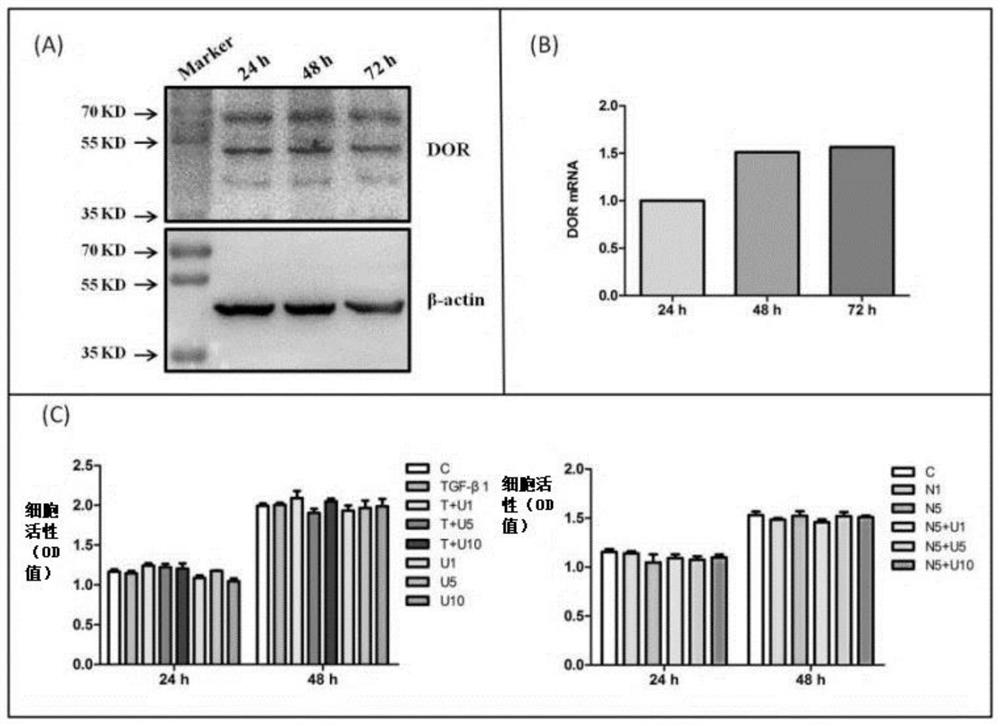
[0062] This example studies the effects of TGF-β1 and DOR activation on NRK-52E cells. Specific experimental results such as figure 1 Shown in the figure (A): DOR expression in NRK-52E cells. Western blotting was used to detect the expression of DOR in these cells (24-72h). (B): mRNA level of DOR in NRK-52E cells. (C): Effect of TGF-β1, UFP-512 and Naltrindole on cell viability. 10ng / ml of specific DOR agonist UFP-512 at different concentrations (0, 1, 5, 10 μM) was added to the culture medium for 24-48h, and the cell viability was measured with a cell counting kit (CCK 8). Among them, C: control group. T: 10 ng / ml TGF-β1. U: UFP-512. N: Naltrindole (inhibitor of DOR). T+U1: 10 ng / ml TGF-β1 + 1 μM UFP-512. T+U5: 10 ng / ml TGF-β1 + 5 μM UFP-512. T+U10: 10 ng / ml TGF-β1 + 10 μM UFP-512. N1: 1 μM Naltrindole. N5: 5 μM Naltrindole. N5+U1: 5 μM Naltrindole + 1 μM UFP-512. N5+U5: 5 μM Naltrindole + 5 μM UFP-512. N5+U10: 5 μM Naltrindole + 10 μM UFP-512.
[0063] figure...
Embodiment 2
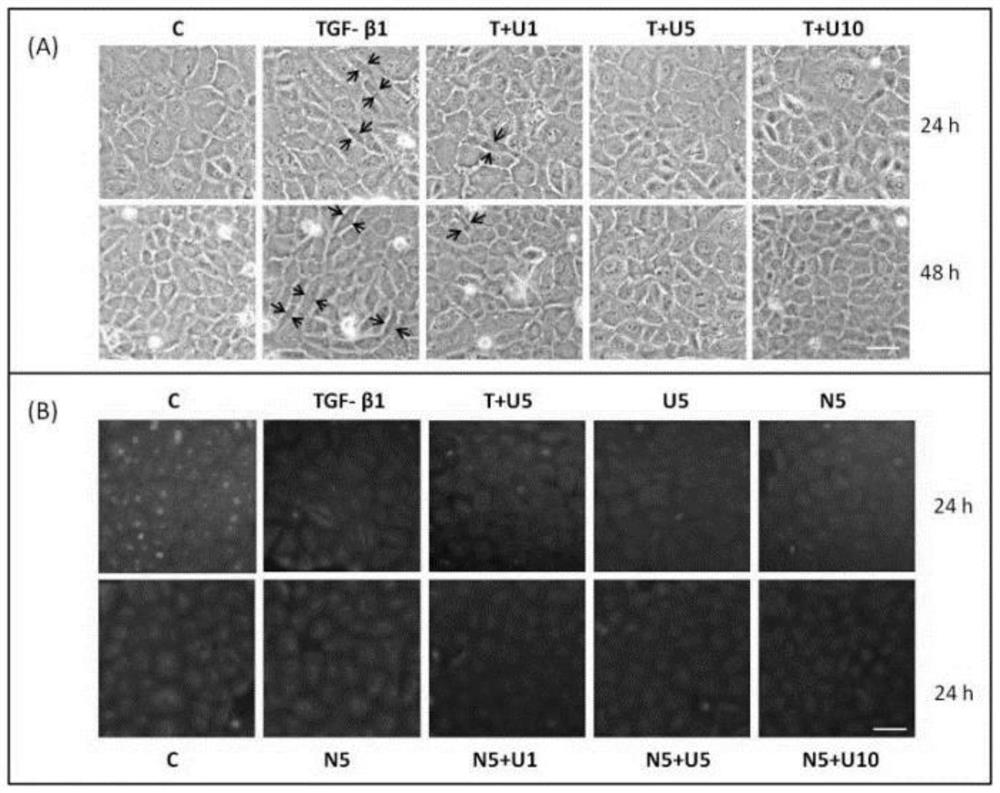
[0066] This example studies the effect of DOR activation on TGF-β1-induced morphological changes. The result is as figure 2 As shown in the figure: (A): The effect of TGF-β1 and UFP-512 on cell morphology changes under light microscope. NRK-52E cells were incubated with 10ng / ml TGF-β1 for 24-48h, different concentrations (0, 1, 5, 10μM) of UFP-512, and the cell morphology was observed under a light microscope. (B): Immunofluorescent staining of NRK-52E cells. The cells were treated with drugs for 24 hours, and the cell morphology was observed by β-tubulin immunofluorescent staining. Among them, C: control group. T: TGF-β1. U: UFP-512. N: Naltrindole. T+U1: 10 ng / ml TGF-β1 + 1 μM UFP-512. T+U5: 10 ng / ml TGF-β1 + 5 μM UFP-512. T+U10: 10 ng / ml TGF-β1 + 10 μM UFP-512. N5+U1: 5 μM Naltrindole + 1 μM UFP-512. N5+U5: 5 μM Naltrindole + 5 μM UFP-512. N5+U10: 5 μM Naltrindole + 10 μM UFP-512.
[0067] The results showed that the NRK-52E cells in the control group (untreate...
Embodiment 3
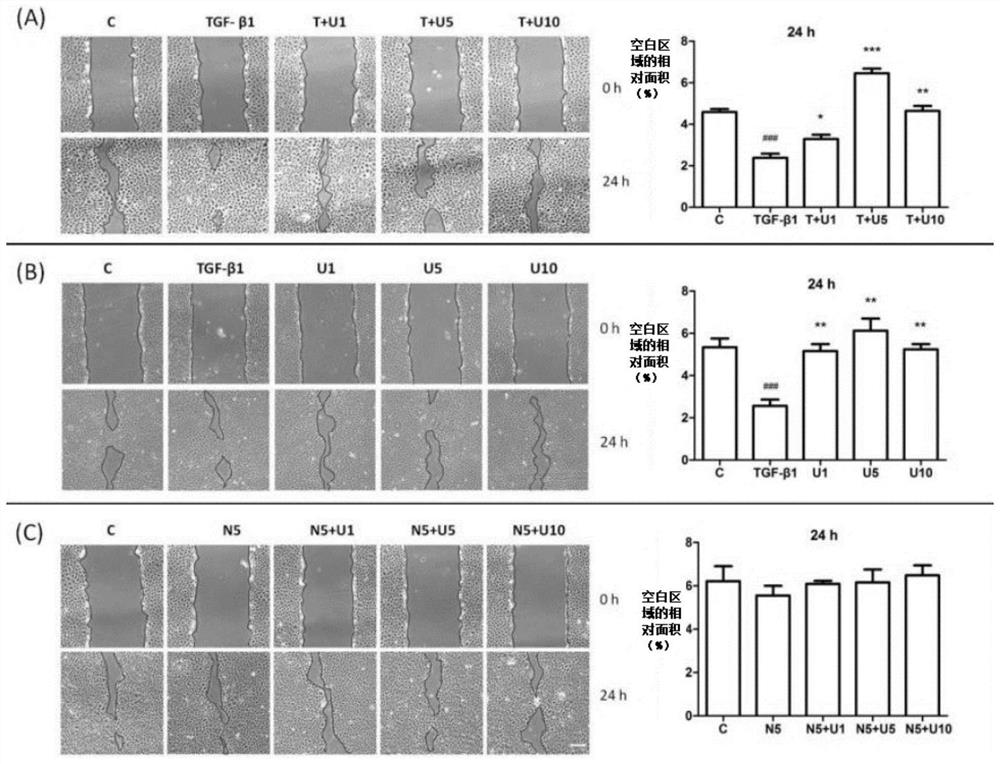
[0069] This example studies the effect of DOR activation on cell migration induced by TGF-β1. The result is as image 3 Shown, (A): Effects of TGF-β1 and UFP-512 on cell migration. (B): Effect of DOR activation or inactivation on cell migration. (C): Effect of Naltrindole and UFP-512 on cell migration. The NRK-52E cell layer on the culture dish was scraped off with a micropipette and observed under a microscope before and after 24 hours of drug treatment. Among them, C: control group. T: TGF-β1. U: UFP-512. N: Naltrindole. T+U1: 10 ng / ml TGF-β1 + 1 μM UFP-512. T+U5: 10 ng / ml TGF-β1 + 5 μM UFP-512. T+U10: 10 ng / ml TGF-β1 + 10 μM UFP-512. N5+U1: 5 μM Naltrindole + 1 μM UFP-512. N5+U5: 5 μM Naltrindole + 5 μM UFP-512. N5+U10: 5 μM Naltrindole + 10 μM UFP-512.
[0070] The results showed that TGF-β1 treatment increased cell migration, while UFP-512 reversed the effect of TGF-β1. On the other hand, UFP-512 or / and Naltrindole had no significant effect on cell migration ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com