Application of BIIB021 in preparation of medicine for preventing and/or treating adenovirus infection
A 1. BIIB021, adenovirus technology, applied in the field of medicine, can solve problems such as undiscovered, achieve the effect of inhibiting replication, wide application prospects, and short drug cycle
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Evaluation of Cytotoxicity of BIIB021 in Vero Cell Line
[0058] 1 cell culture
[0059] After 2 passages of cryopreserved and resuscitated cells, use DMEM medium containing 10% fetal bovine serum and double antibody (penicillin 100U / ml, streptomycin 100μg / ml) for expansion and culture, and the seeding density is not less than 1 ×10 4 cell / ml, passage density not higher than 5×10 4 cells / ml.
[0060] 2 Drug-treated cells
[0061] Vero cells by 1 x 10 4 Cells / well (volume 100 μL) were seeded in 96-well cell culture plates, and cultured for 24 hours until the confluence of cell wells reached 80%; 200 μL of medium (DMEM medium + 2% serum + double antibody) was used in each well to prepare drugs, and Add to the corresponding cell wells and mix. Seven concentration gradients were set for the drug, and two duplicate wells were set for each gradient concentration, and the final concentrations were 0.007 μM, 0.02 μM, 0.06 μM, 0.19 μM, 0.56 μM, 1.67 μM and 5 μM,...
Embodiment 2
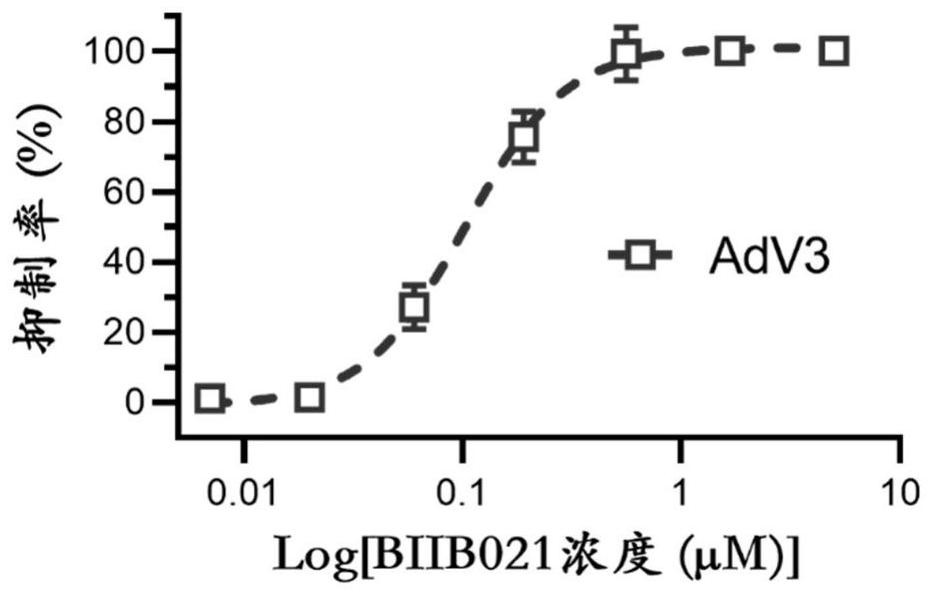
[0066] Example 2: Evaluation of anti-AdV3 adenovirus activity of BIIB021 in Vero cell line
[0067] 1 cell culture
[0068] After 2 passages of cryopreserved and resuscitated cells, use DMEM medium containing 10% fetal bovine serum and double antibody (penicillin 100U / ml, streptomycin 100μg / ml) for expansion and culture, and the seeding density is not less than 1 ×10 4 cell / ml, passage density not higher than 5×10 4 cells / ml.
[0069] 2 Drug-treated cells
[0070] Vero cells by 1 x 10 4 Cells / well (volume 100μL) were inoculated in 96-well cell culture plates, and cultured for 24h until the confluence of cell wells reached 80%; the infection group was added with 0.55MOI (multiplicity of infection) AdV3 virus, and at the same time, various gradient concentrations of drugs (at 5μM) were added. As the starting concentration, serially diluted 7 gradients by 3 times, with two duplicate wells for each gradient) to a total volume of 200 μL of culture medium (DMEM medium + 2% seru...
Embodiment 3
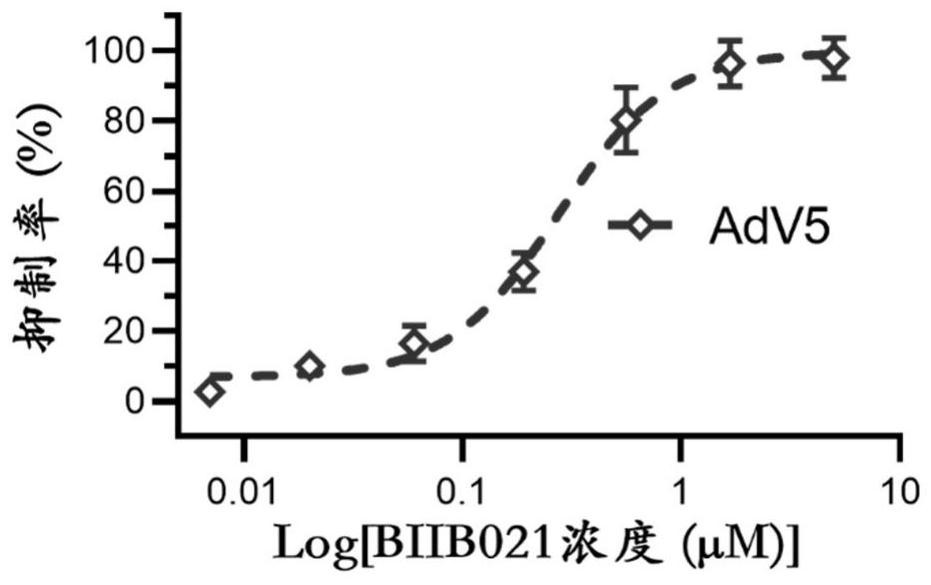
[0080] Example 3: Evaluation of anti-AdV5 adenovirus activity of BIIB021 in Vero cell line
[0081] 1 cell culture
[0082] After 2 passages of cryopreserved and resuscitated cells, use DMEM medium containing 10% fetal bovine serum and double antibody (penicillin 100U / ml, streptomycin 100μg / ml) for expansion and culture, and the seeding density is not less than 1 ×10 4 cell / ml, passage density not higher than 5×10 4 cells / ml.
[0083] 2 Drug-treated cells
[0084] Vero cells were inoculated into 96-well cell culture plates at 1×104 cells / well (volume 100 μL), and cultured for 24 hours until the confluence of cell wells reached 80%; the infection group was added with 1.1 MOI (multiplicity of infection) of AdV5 virus, and each gradient was added at the same time. The concentration of the drug (starting at 5 μM, serially diluted 3-fold in 7 gradients, each gradient is two replicates) to a total volume of 200 μL of culture medium (DMEM medium + 2% serum + double antibody), in ce...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Passage density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com