Radioactive resin microsphere injection as well as preparation method and application thereof
A resin microsphere, radioactive technology, applied in the field of medicine, can solve the problem of inability to determine the dose of radioactive administration, and achieve the effect of solving difficult direct injection and reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
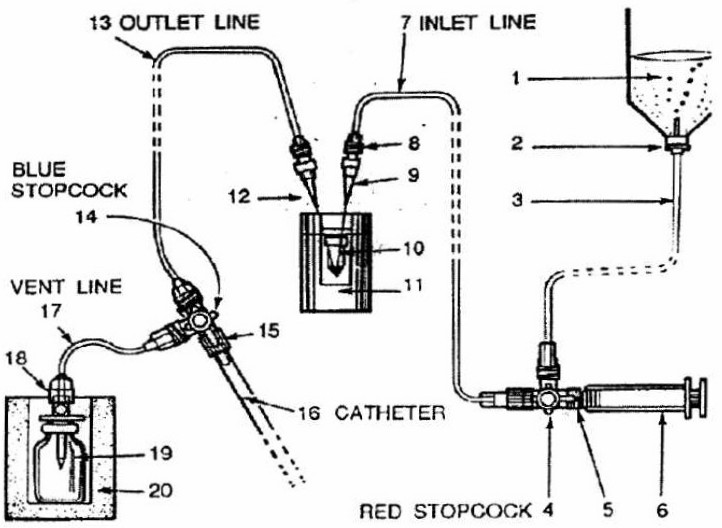
Image
Examples
Embodiment 1
[0048] A preparation of yttrium [ 90 Y] the method for resin microsphere injection, this method comprises the following steps:
[0049] Dissolve 20 mg of sodium alginate in 10 ml of sterile water for injection, pass through a 0.22 μm sterile filter membrane, add 10 ml of 0.45 g / L sterile calcium chloride solution, and mix to obtain a viscosity of 16.6 mPa·S (about 20 ℃) calcium alginate hydrogel, as A1 agent; the yttrium [ 90 50mg of Y] resin microspheres were dispersed in 1ml of sterile water for injection, packaged, and sterilized by moist heat at 121°C for 15min for later use. The radioactive resin microspheres did not start to settle within 2 hours of the injection.
Embodiment 2
[0051] A preparation of yttrium [ 90 Y] the method for resin microsphere injection, this method comprises the following steps:
[0052] Dissolve 20 mg of sodium alginate in 10 ml of sterile water for injection, pass through a 0.22 μm sterilizing filter membrane, and encapsulate it for later use as agent A2. The yttrium [ 90 Y] 100 mg of resin microspheres are dispersed in 1 ml of sterile water for injection containing 5 mg of calcium chloride, packaged, sterilized by moist heat at 121°C for 15 minutes, and used as agent B2. Add the A2 agent obtained above to the B2 agent, and mix well. The radioactive resin microspheres did not start to settle within 2 hours of the injection.
Embodiment 3
[0054] A preparation of holmium [ 166 Ho] the method for resin microsphere injection, the method comprises the following steps:
[0055] Dissolve 20 mg of sodium alginate in 10 ml of sterile water for injection, pass through a 0.22 μm sterilizing filter membrane, and encapsulate it for later use as agent A2. The holmium [ 166 300 mg of Ho] resin microspheres were dispersed in 1 ml of sterile water for injection containing 5 mg of calcium chloride, packaged, sterilized by moist heat at 121°C for 15 minutes, and used as agent B2. Add the A2 agent obtained above to the B2 agent, and mix well. The radioactive resin microspheres did not start to settle within 2 hours of the injection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com