Synthesis method of oseltamivir intermediate
A technology of oseltamivir and a synthesis method, which is applied in the field of synthesis of oseltamivir intermediates, can solve the problems of long route, unsuitability for scale-up production, low yield and the like, shorten the reaction route, improve drug safety, The effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
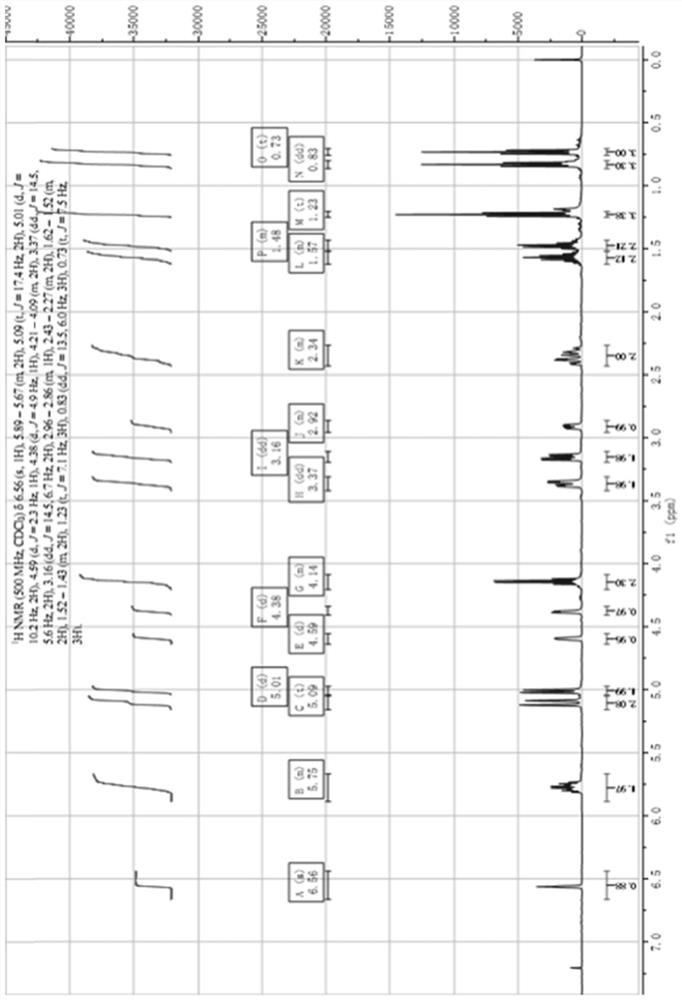
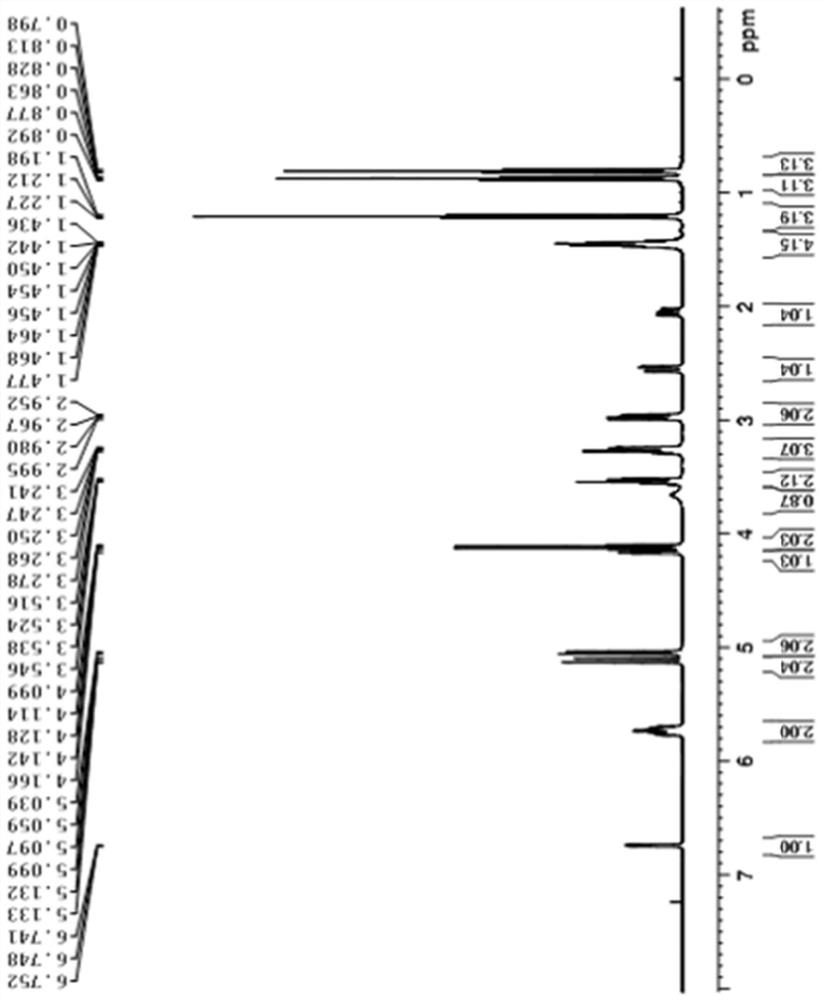
Image
Examples
Embodiment 1
[0053] A kind of synthetic method of oseltamivir intermediate, its synthetic route is:
[0054]
[0055] The specific synthetic method comprises the following steps:
[0056](1) in 100L reactor, add 10kg compound 1 and 30L methylene chloride, drum nitrogen protection, about -10 ℃ of refrigerant temperature control, add 12kg trifluoromethanesulfonic anhydride under stirring, keep low temperature and slowly drip triethylamine 6kg, Incubate and stir for about 2 hours, wash with water after the reaction, and concentrate at room temperature until no liquid flows out to obtain a viscous liquid reaction mixture. To the reaction mixture was added 5 kg of diallylamine, 30 L of toluene with stirring, and the temperature was raised to 80°C. Start timing when the temperature reaches 80°C and stir for about 8 hours. After the reaction was completed, the temperature was cooled to below 20° C., 20 L of toluene was added and stirred for 30 minutes, 30 L of 5% citric acid aqueous solution...
Embodiment 2
[0066] A kind of synthetic method of oseltamivir intermediate, comprises the steps:
[0067] (1) Inject 10kg of compound 1 and 40L of methylene chloride into a 100L reaction kettle, drum nitrogen protection, and control the temperature of the refrigerant at about -20°C. 12kg of trifluoromethanesulfonic anhydride was added under stirring, and 8kg of diisopropylethylamine was slowly added dropwise at a low temperature, and the mixture was kept stirring for about 4 hours. After the reaction, wash with water, and concentrate at room temperature until no liquid flows out to obtain a viscous liquid reaction mixture. To the reaction mixture was added 5 kg of diallylamine, 30 L of toluene with stirring, and the temperature was raised to 90°C. When the temperature reached 90° C., the timer was started, and the mixture was stirred for about 20 hours. After the reaction was completed, the temperature was cooled to below 20° C., 20 L of toluene was added and stirred for 30 minutes, 30 L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com