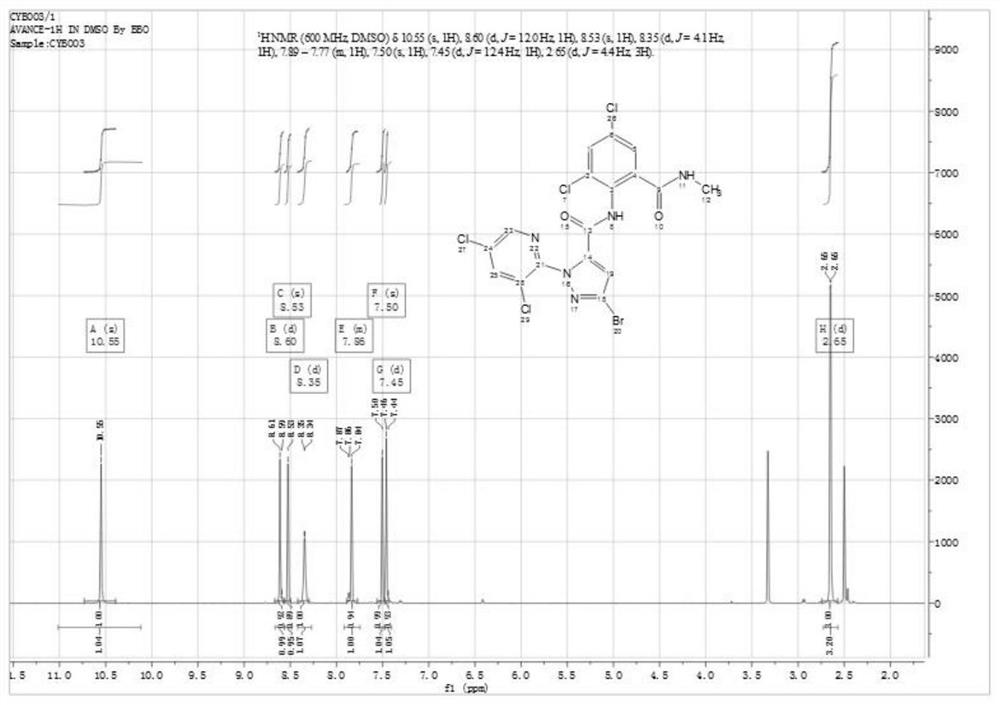
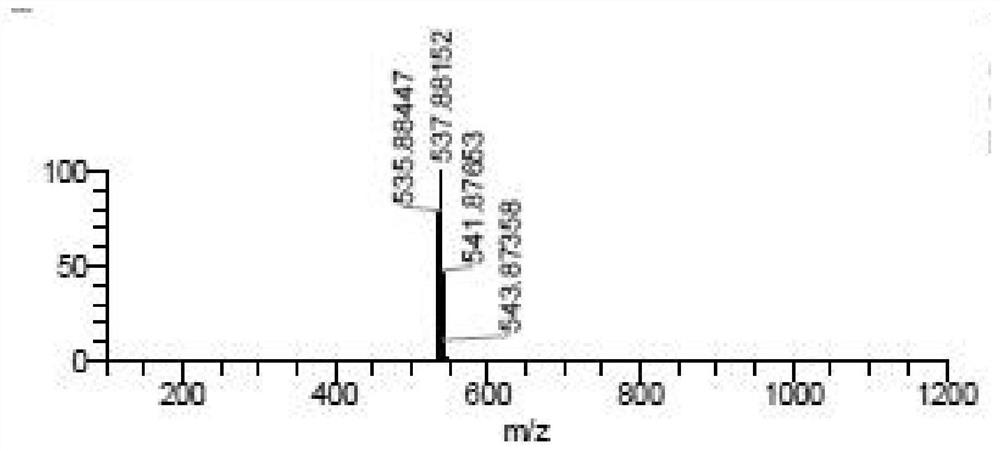
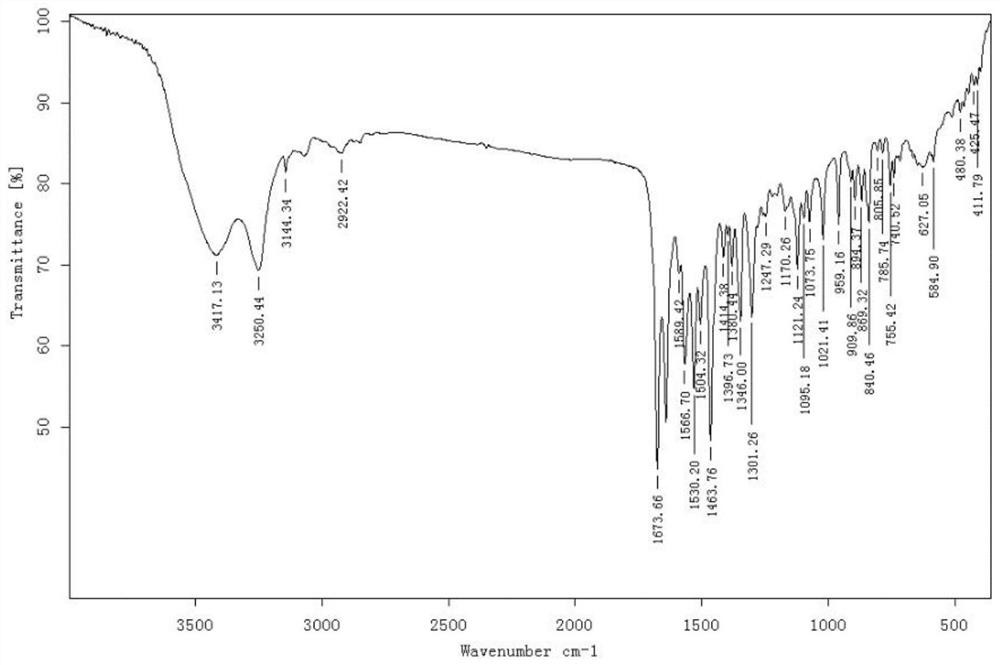
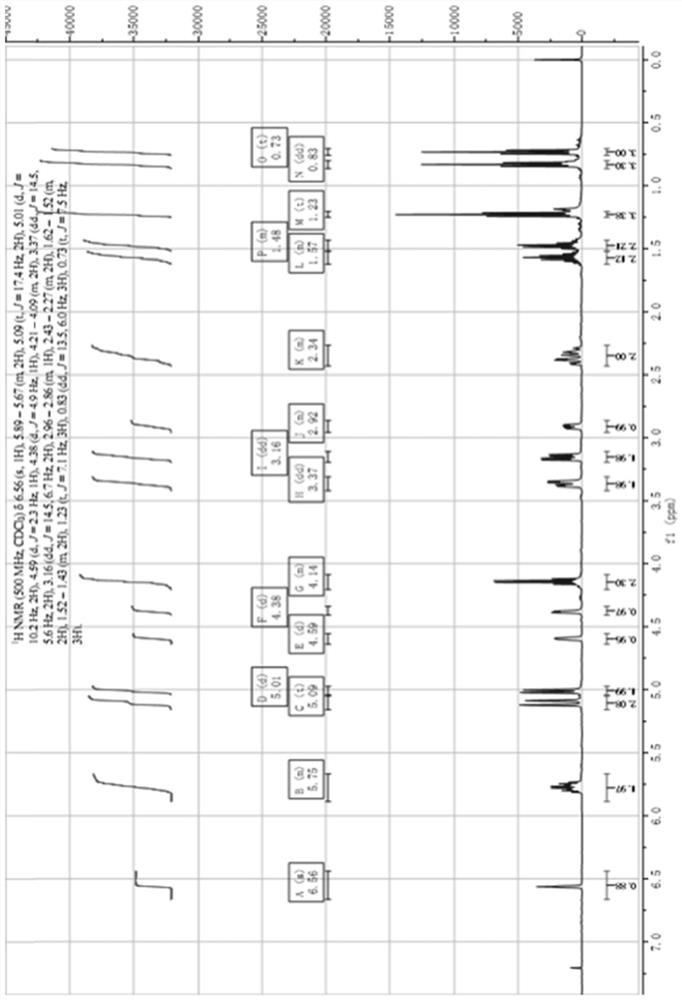
Patents
Literature
63 results about "Mesitoyl chloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Preparation method of trifluoro methanesulfonic anhydride
ActiveCN102911086AHigh yieldReduce contentOrganic chemistryOrganic compound preparationTrifluoromethanesulfonic anhydrideSulfonyl chloride
The invention provides a preparation method of trifluoro methanesulfonic anhydride. The method comprises the following steps of: firstly reacting trifluoro methanesulphonyl fluoride with alkali metal hydroxide to prepare trifluoro mesylate, purifying trifluoro mesylate by recrystallization by utilizing an organic solvent, reacting trifluoro methane sulfonyl chloride with trifluoro mesylate to generate a trifluoro methanesulfonic anhydride crude product, and finally purifying trifluoro methanesulfonic anhydride by atmospheric distillation. The preparation method of trifluoro methanesulfonic anhydride can be used for not only effectively simplifying reaction steps so that the operation process is simple and convenient and the operation is safe, but also avoiding byproducts generated in the process of the traditional method for producing trifluoro methanesulfonic anhydride, and effectively reducing the contents of F<-> and SO4<2-> in the product; by utilizing recrystallization, atmospheric distillation and other methods for purification, the product purity is up to 99.5%; and more importantly, the yield of anhydride is greatly increased and raised to 88% from original 60%.
Owner:JIANGXI GUOHUA IND CO LTD
Suppression of carcinoma using high purity conjugated fatty acid
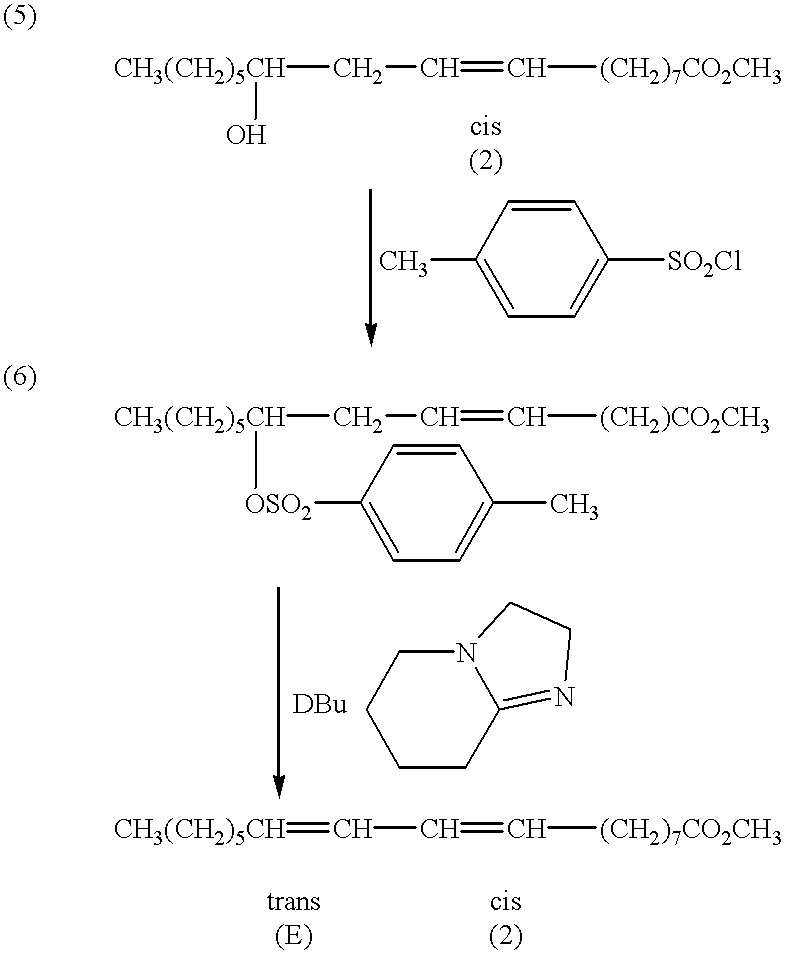
Treatment of carcinoma in a human is disclosed, including administering a therapeutically effective amount of a fatty acid having four carbons with two conjugated double bonds formed by reacting an ester of a fatty acid with a tosyl chloride or a mesyl chloride, the fatty acid having four carbon atoms such that carbon one bears one hydrogen and one hydroxyl group, carbon two bears two hydrogens, and a double bond is positioned between carbon three and four. The tosylate or mesylate of the ester of the fatty acid having the chain of four carbon atoms such that carbon one bears one hydrogen and one hydroxyl group, carbon two bears two hydrogens, and a double bond is positioned between carbon three and four is reacted with diazabicyclo-undecene. The method includes administering to a human a highly purified fatty acid in accordance with the present invention.
Owner:MATREYA
Preparation method of azacyclo
The invention discloses a preparation method of azacyclo. The preparation method comprises the following steps of: (1) reacting diethylenetriamine or triethylene tetramine with methylsulfonyl chloride to generate methanesulfonamide; (2) during two-phase reaction, cyclizing compound catalytic cyclization on methanesulfonamide and a compound having the structure shown in a chemical formula (a) under the composite catalysis of benzyl triethylanmine compound and 15-crown-5 at 90 DEG C under a backflow condition; and (3) removing methylsulfonyl from methanesulfonamide azacyclo and performing methylation treatment, wherein X represents bromine, iodine or sulphonate. The preparation method has the advantages that raw materials are low in cost, atom economy is high, and operation is easy and safe. The preparation method is suitable for industrial production.
Owner:浙江凯普化工有限公司
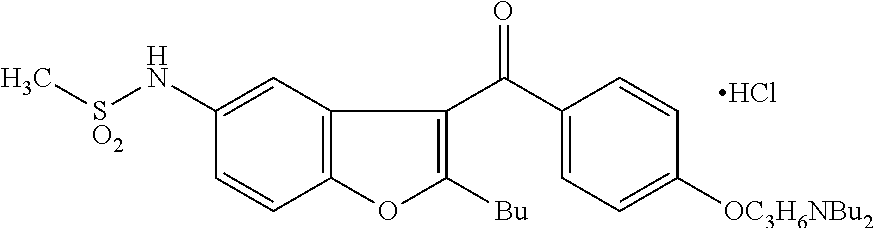
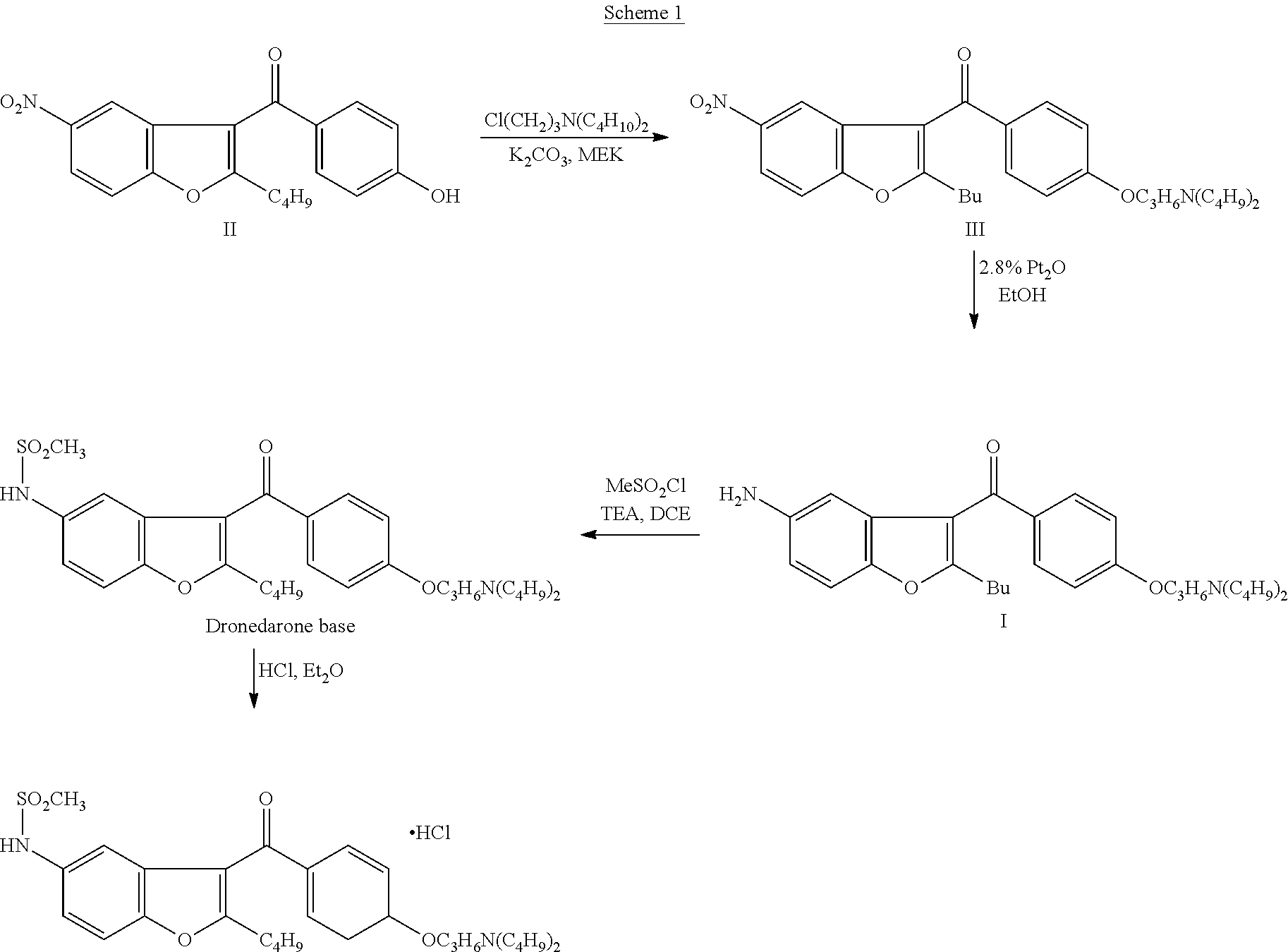
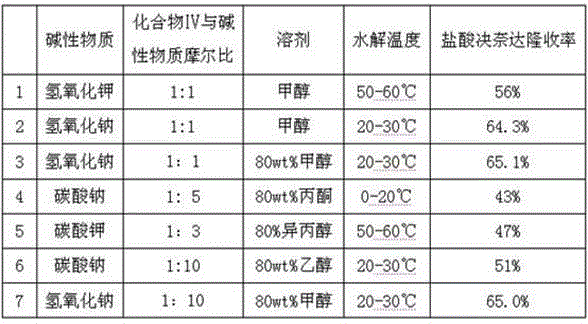
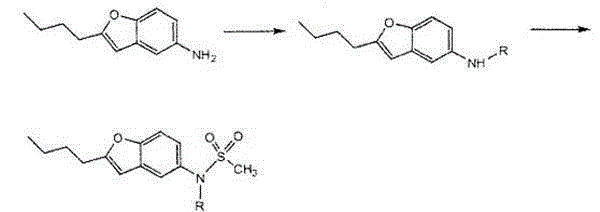
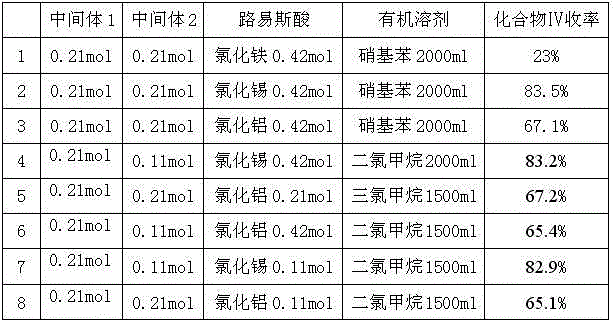
Preparation method of dronedarone hydrochloride and intermediate of dronedarone hydrochloride
InactiveCN102675267AInhibit side effectsImprove responseOrganic compound preparationAmino-hyroxy compound preparationPtru catalystAcyl group
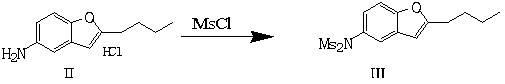
The invention discloses a preparation method of an intermediate of dronedarone hydrochloride, which comprises the step of reacting 2-butyl-5-amino benzofuran hydrochloride serving as a starting material with methylsulfonyl chloride in the presence of a basic catalyst and an organic solvent to produce the intermediate of dronedarone hydrochloride, wherein the intermediate is 2-butyl-5-((N, N-dimethyl sulfonyl) amido) benzofuran. The invention also discloses a method for preparing dronedarone hydrochloride by using the intermediate. The preparation of the intermediate is simple and easy to operate; amino is protected by methylsulfonyl, so that the side reaction possibly generated in the reaction for preparing the dronedarone hydrochloride at the later period can be avoided, and the difficulty in purification at the later period can be simplified; the dronedarone hydrochloride yield is high; the problems in the prior art that the preparation of dronedarone hydrochloride is complicated with high cost, the intermediate is difficult to purify and aftertreatment is complicated can be solved; and the preparation difficulty of dronedarone hydrochloride is greatly reduced.
Owner:山东富创医药科技有限公司
Ratio type two-photon fluorescent probe as well as preparation method and application thereof
ActiveCN111875560ADeep penetrationAvoid damageOrganic chemistryFluorescence/phosphorescenceFluoProbesHexamethylenetetramine
The invention belongs to the technical field of synthesis and fluorescence detection, and discloses a ratio type two-photon fluorescence probe as well as a preparation method and application thereof.The preparation method of the fluorescent probe comprises the following steps: reacting hydroxyl tetraphenyl ethylene with hexamethylenetetramine to obtain a compound 1, reacting the compound 1 with o-aminothiophenol to obtain a compound 2, and adding trifluoromethanesulfonyl chloride into the compound 2 to generate a final product, namely the ratio type two-photon fluorescent probe TPEBTTF. Whenthe fluorescent probe is applied to detection of superoxide anions, the concentration of the superoxide anions can be determined, and the selectivity is high; in addition, the fluorescent probe has atwo-photon excitation characteristic, can perform ratio two-photon fluorescence imaging detection on superoxide anions in samples such as cells by means of a two-photon confocal microscope, and has agood imaging effect.
Owner:SHANDONG NORMAL UNIV
Synthesis method of trimethoxystilbene
InactiveCN105693477ALow costEasy to operateSulfonic acid amide preparationSulfonic acid preparationTriflic acidProcess engineering
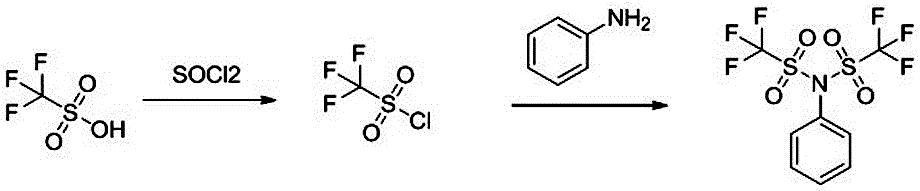
The invention discloses a synthesis method of trimethoxystilbene. The method comprises the following steps of (1) at room temperature, adding trifluoromethanesulfonic acid into dichloromethane; stirring the mixture; slowly dripping sulfoxide chloride; after the dripping is completed, performing stirring reaction for 12 hours at room temperature; concentrating a reaction system to obtain a coarse product of trifluoromethanesulfonyl chloride; (2) adding aniline and triethylamine into dichloromethane; lowering the temperature to 0 DEG C; dripping the obtained coarse product of trifluoromethanesulfonyl chloride through a dropping funnel; after the dripping is completed, raising the temperature to room temperature for reaction for 2 hours; performing water washing, drying and concentration on the reaction system; then, performing recrystallization by petroleum ether to obtain trimethoxystilbene. The synthesis method has the beneficial effects that the raw materials of trifluoromethanesulfonic acid used by the synthesis method of trimethoxystilbene provided by the invention are common chemical raw materials; the price is low; the acquisition is easy; the cost is greatly reduced; special equipment such as low-temperature kettles is not needed in the production process; the operation is easy; the method is suitable for industrial production.
Owner:BAIYIN HAIRUIDA BIOCHEM TECH CO LTD
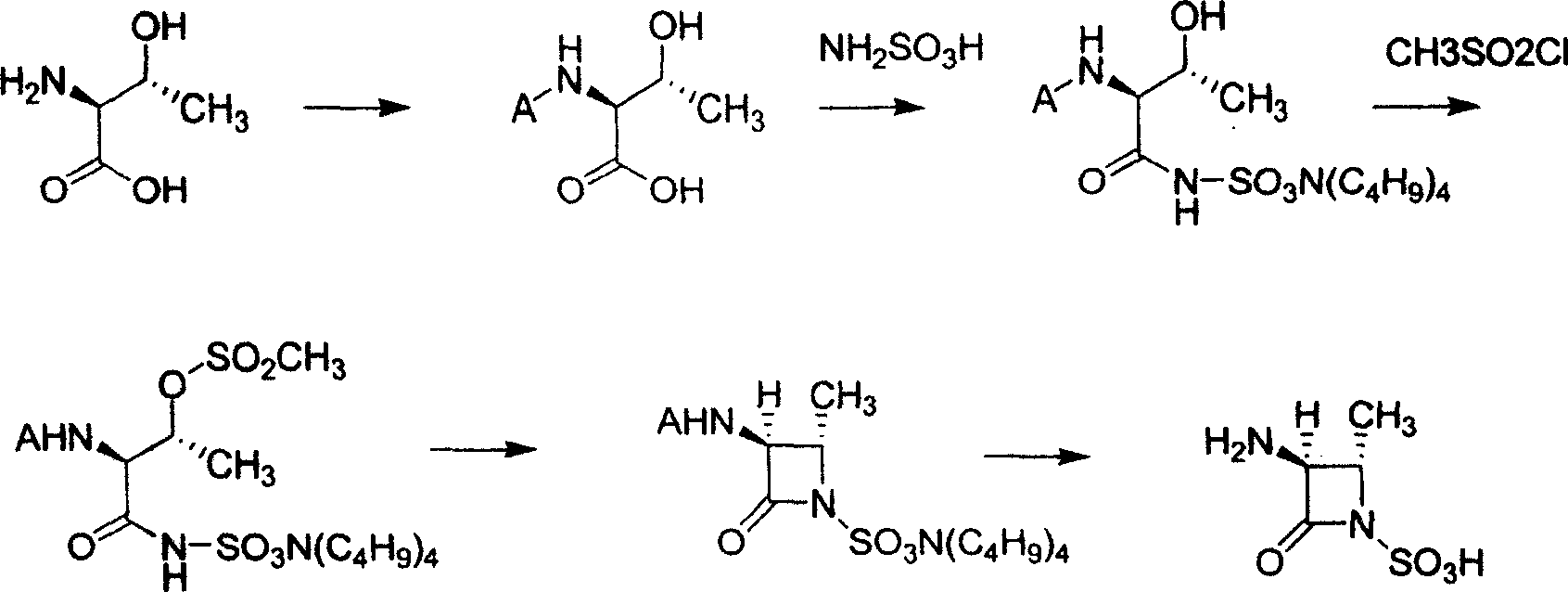
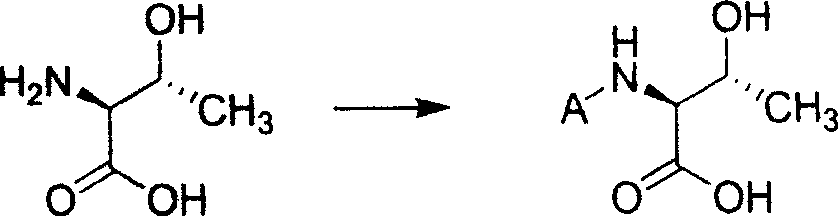
Process of synthesizing (2S-trans)-3-methyl-4-oxo-1-azacyclo butyl sulfonic acid
The present invention relates to process of synthesizing (2S-trans)-3-amino-2-methyl-4-oxo-1-azacyclo butyl sulfonic acid. The process includes the following steps: 1. reaction of L-threonine and matter to protect its amino group; 2 condensation of L-threonine with protected amino group and aminosulfonic acid to obtain threonyl aminosulfonic acid with protected amino group; 3. reaction of threonyl aminosulfonic acid with protected amino group and methylsulfnyl chloride to protect hydroxyl radicap in threonine segment; 4. cyclization reaction to form protected quaternary ammonium salt of azacyclo butyl sulfonic acid; and 5. deprotection to obtain (2S-trans)-3-amino-2-methyl-4-oxo-1-azacyclo butyl sulfonic acid.
Owner:CHINA RESOURCES SAIKE PHARMA
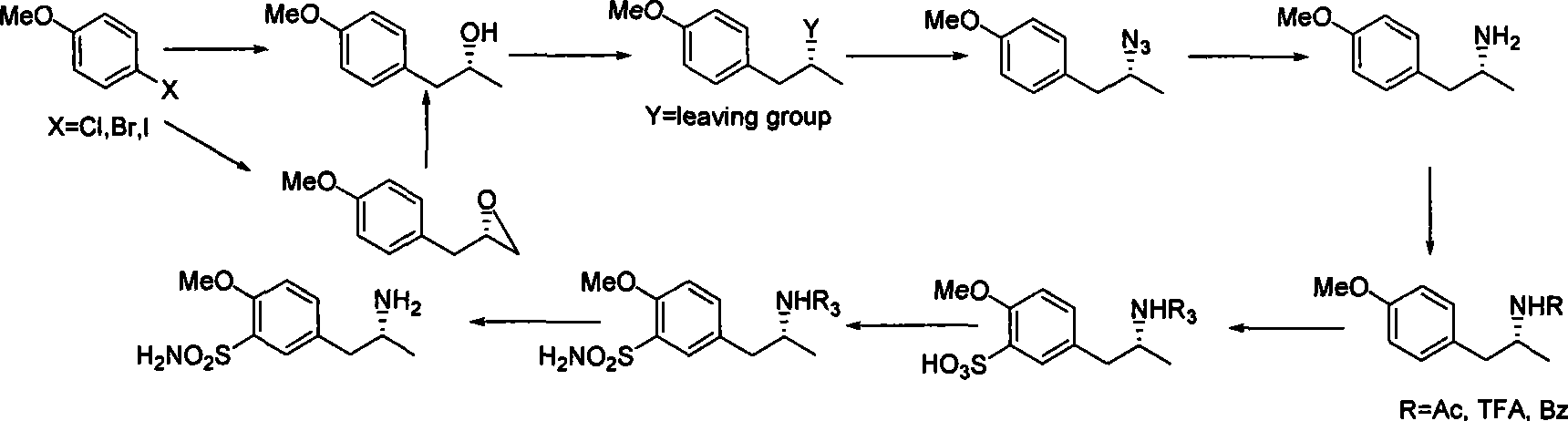
Method for synthesizing (R)-5-(2-amino propyl)-2-methoxybenzenesulfonamide
InactiveCN101462986ALow priceEasy to crystallize and purifySulfonic acid amide preparationEpoxyLeaving group
The invention relates to a method for synthesizing (R)-5-(2-aminopropyl)-2-methoxyl benzsulfamide. In the method, dihalogen anisole is used as initial raw material to react with metal. The reaction for opening ring of chiral propylene oxide or chiral epoxy chloropropane is the key reaction. Newly generated hydroxide radical reacts with substituted benzene sulfone chloride or methylsulfonyl chloride to generate leaving group to be ammonolyzed or azided and hydrolyzed or reduced into amine. (R)-5-(2-aminopropyl)-2-methoxyl benzsulfamide is prepared by protecting amido, benzene ring sulfonation, acylatation, aminolysis and deamination protection.
Owner:SHANGHAI BAILING PHARMA TECH CO LTD +1
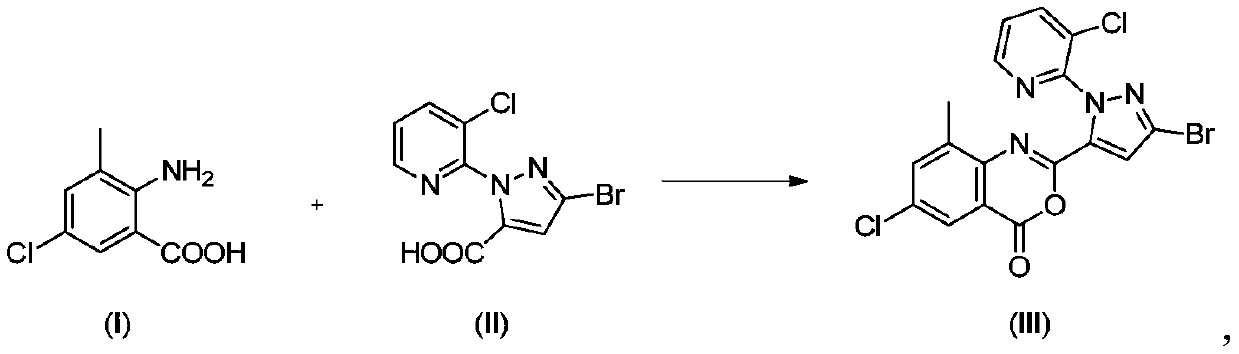
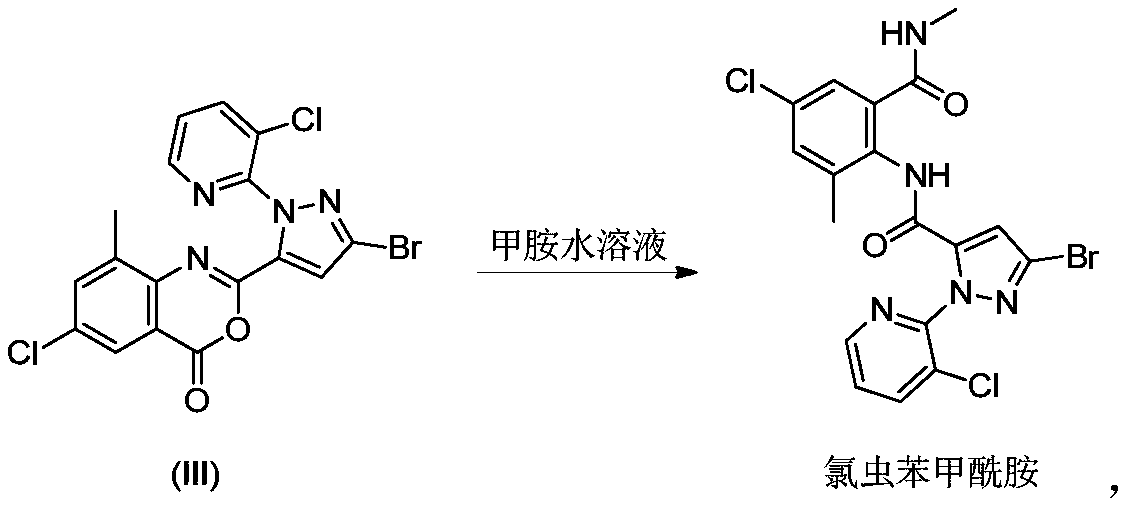
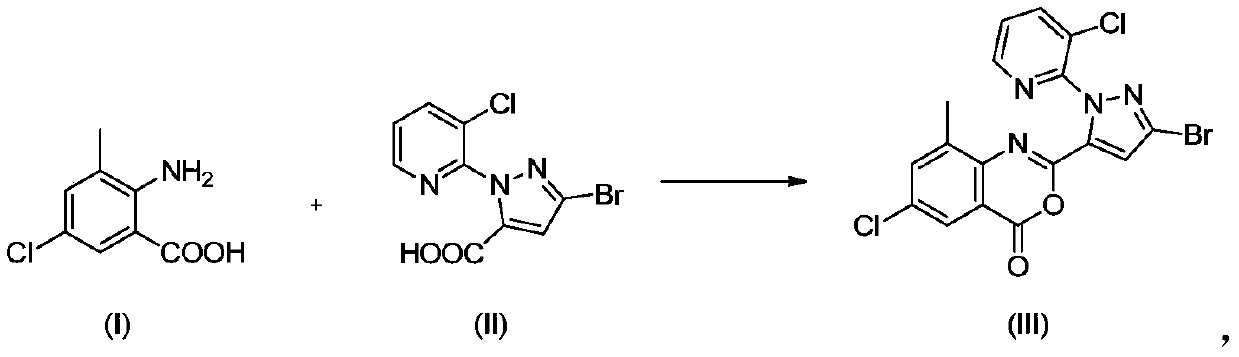
Preparation method of chlorantraniliprole and intermediate thereof
The invention discloses a preparation method of chlorantraniliprole and an intermediate of chlorantraniliprole, which comprises the following steps: in the presence of an alkali and a solvent, mixing3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazole-5-carboxylic acid and 2-amino-5-chloro-3-methylbenzoic acid together to form a material I; and mixing the methylsulfonyl chloride with the material I, and carrying out a reaction so as to obtain a 2-[3-bromo-1-(3-chloro-2-pyridyl)-1H-pyrazol-5-yl]-6-chloro-8-methyl-4H-3, 1-benzoxazine-4-one intermediate, i.e., a chlorantraniliprole intermediate. The method is easy to operate, the benzoxazinone intermediate generated in the reaction process does not need to be further treated, can be adopted in a next step of reaction directly without drying for preparation of chlorantraniliprole; the method has the advantages that the method is simple in process and high in total yield and product quality, a filtrate can be used by the aid of synchronous and continuous application processes, accordingly, the usage amount of the solvent can be reduced to a great extent, and the method is suitable for industrial production.
Owner:LIER CHEM CO LTD +1
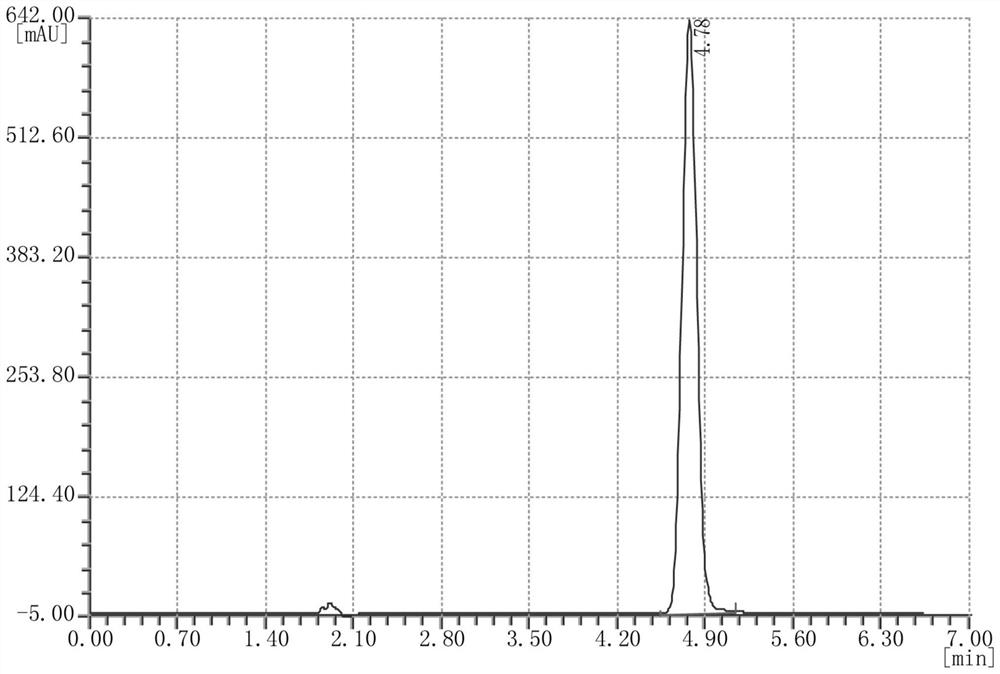
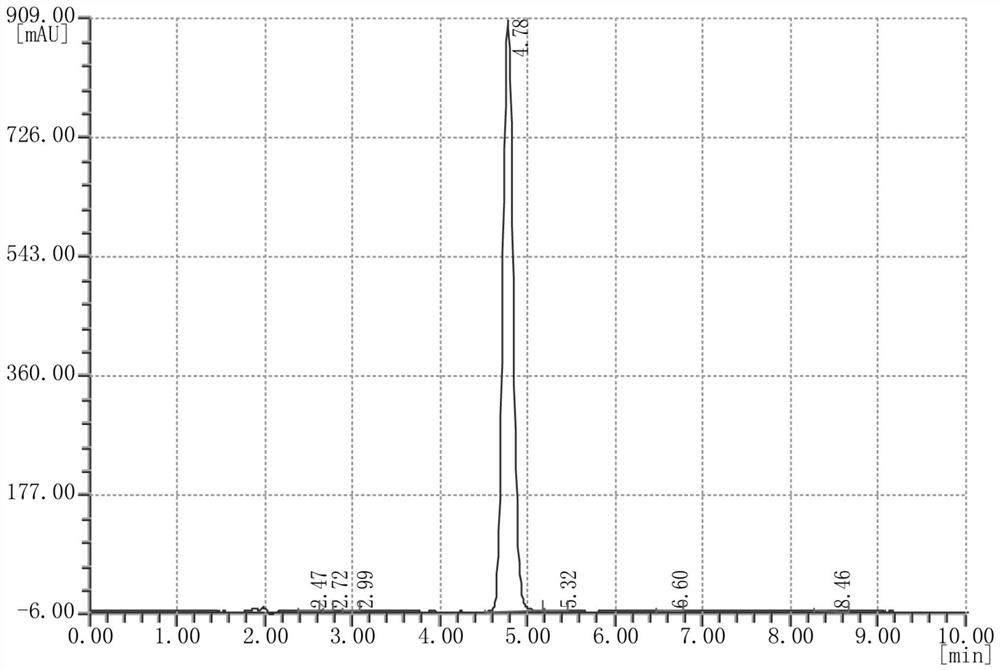
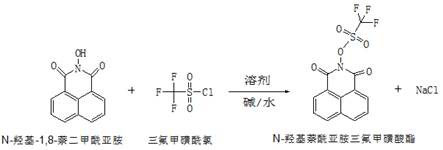
Synthesis method of N-hydroxynaphthalimide trifluoromethanesulfonate
The invention relates to a synthesis method of N-hydroxylnaphthalimide trifluoromethanesulfonate, and belongs to the field of organic synthesis. The method comprises the following steps: (1) taking N-hydroxyl-1,8-naphthalimide and trifluoromethanesulfonyl chloride as raw materials, aromatic hydrocarbon as a solvent and an alkaline solution as a catalyst to carry out esterification reaction, and after the reaction is finished, extracting and concentrating to obtain an N-hydroxylnaphthalimide trifluoromethanesulfonate crude product; and (2) recrystallizing the N-hydroxylnaphthalimide trifluoromethanesulfonate crude product obtained in step (1) to obtain N-hydroxylnaphthalimide trifluoromethanesulfonate, wherein a mixed solution of methylbenzene and water is used as a recrystallization solvent in the recrystallization process. The synthesis method is high in efficiency and high in yield, and the prepared N-hydroxylnaphthalimide trifluoromethanesulfonate is high in purity.
Owner:河北凯力昂生物科技有限公司
Process for obtaining dronedarone
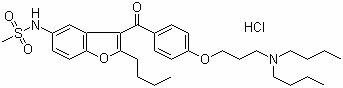
The present invention provides a process for obtaining dronedarone or salts thereof characterized in that in an organic phase comprising one or more non-polar solvents, 5-amino-3-[4-(3-di-n-butylaminopropoxy)benzoyl]-2-n-butyl-benzofuran is reacted with methane sulfonyl chloride without the addition of a base. The invention also provides a process for obtaining intermediates of dronedarone environmentally friendly and industrially viable.
Owner:INKE SA (ES)
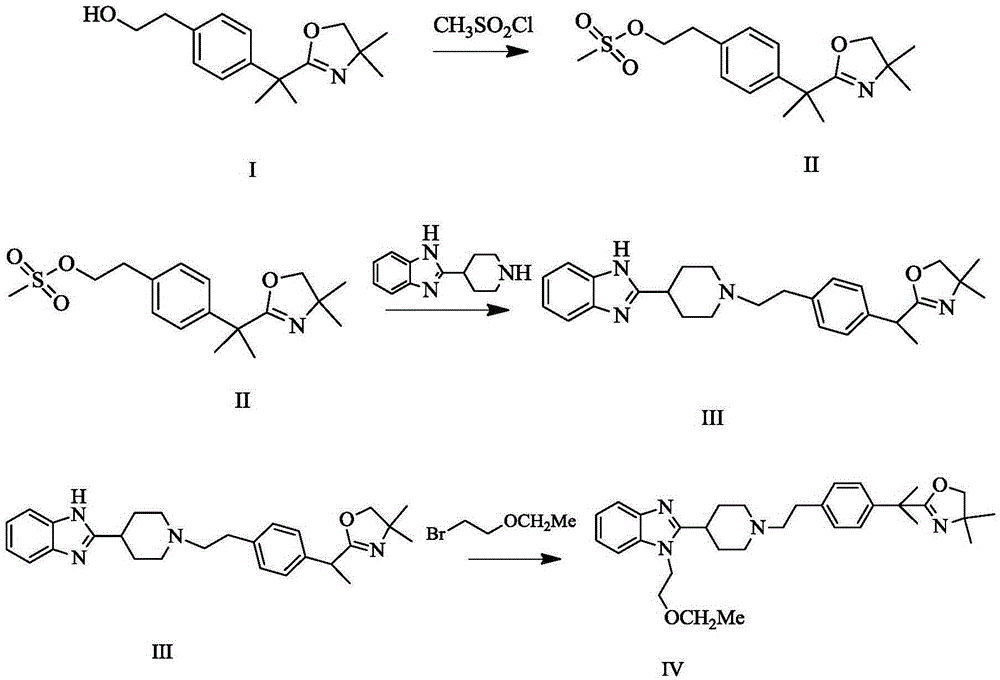
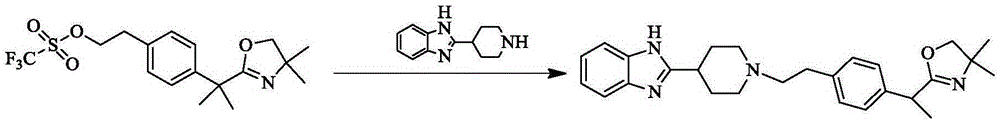
Bilastine intermediate preparation method
The present invention relates to a Bilasiting intermediate 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-2H-oxazol-2-yl)-1-methyl-ethyl]-phenyl}-ethyl)-piperidin-4-yl]-1H-benzimidazole preparation method, the compound 2-{1-[4-(2-hydroxyethyl) phenyl]-1-methylethyl}-4,5-2H-4,4-dimethyl-oxazole is dissolved in an organic solvent, trifluoromethanesulfonyl chloride is dissolved, and added dropwise to the system, after the completion of the addition, triethylamine is added for heating and refluxing for 1h to obtain structure II compound 2-[4-(1-(4,4-dimethyl-2H-oxazol-2-yl)-1-methylethyl) phenyl] ethyl triflate, and the resulting compound 2-[4-(1-(4,4-dimethyl-2H-oxazol-2-yl)-1-methylethyl) phenyl] ethyl triflate is dissolved in an organic solvent, under nitrogen protection, sodium methoxide is added for reaction for 2 h, 2-(4-piperidine)-1 H-benzimidazole is added for heating and refluxing for 2h to obtain 2-[1-(2-{4-[1-(4,4-dimethyl-4,5-2H-oxazol-2-yl)-1-methyl-ethyl]-phenyl}-ethyl)-piperidin-4-yl]-1H-benzimidazole.
Owner:万全万特制药江苏有限公司
Substituted piperazine N-ethyl sulfonamide derivative and preparation and application thereof
InactiveCN101851199AImportant biological activityOrganic active ingredientsOrganic chemistrySulfonyl chloridePositive control
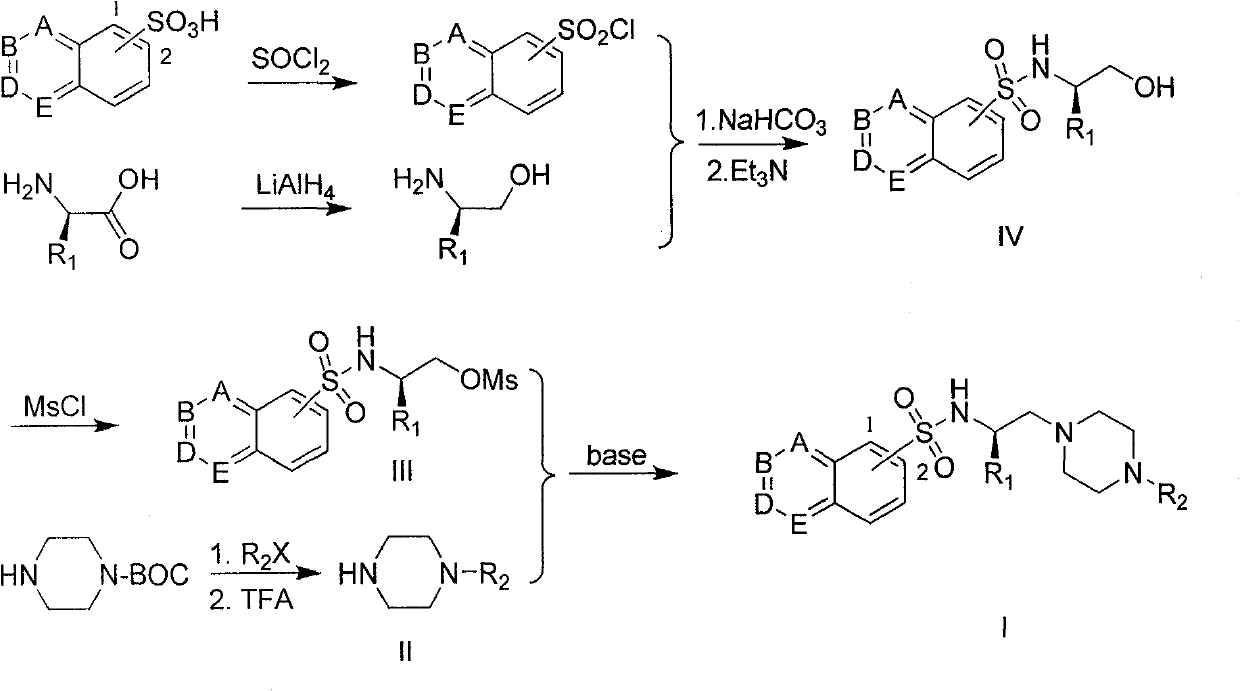
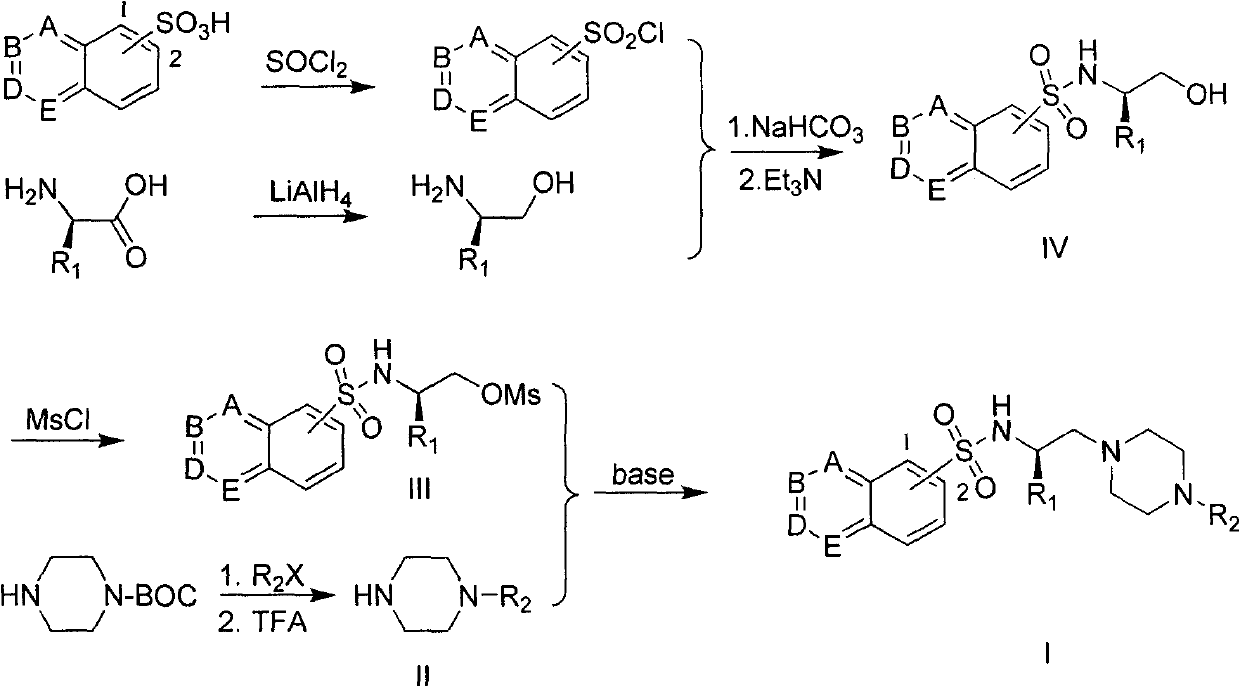
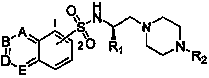
The invention provides a substituted piperazine N-ethyl sulfonamide derivative and medicinal salt thereof. The acyl chloride derivative thereof is obtained by chlorination of substituted sulfoacid by thionyl chloride; substituted L-type amino acid is reduced by lithium aluminium hydride reduction to obtain L-type alkamines compound; the L-type alkamines compound and the substituted sulfoacid derivative are carried out with coupling reaction and protected by mesyl chloride to obtain methanesulfonate derivative; mono-Boc protection piperazine is performed with substitution reaction and trifluoroacetic acid deprotection to obtain monosubstitution piperazine; and the monosubstitution piperazine is carried out with substitution reaction under the catalysis of organic amine to obtain the substituted piperazine N-Ethyl sulfonamide derivative. The virus activity test to five tumour cells in vitro by the compound provided by the invention shows that the in vitro virus activity test of the derivative shows the activity of parts of compound is higher than or comparative to positive control antitumor drug. The invention can be applied in preparing drug for preventing and curing tumour. The general formula of the compound of the invention is shown in the specification.
Owner:ZHEJIANG UNIV
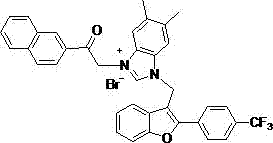
2-aryl-3-methyl benzofuran-benzimidazole salt compound and preparation method thereof
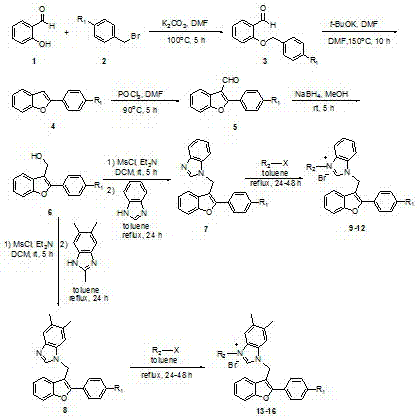
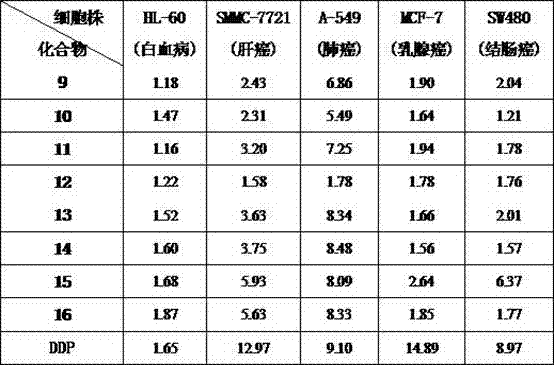
The invention discloses a series of 2-aryl-3-methyl benzofuran-benzimidazole salt compound with a structure general formula (such as formula I or II) and a preparation method thereof. The preparation method comprises the following steps: taking salicylic aldehyde as a raw material and synthesizing benzylide salicylic aldehyde with 4-substituted benzyl bromide in anhydrous DMF; then synthesizing 2-aryl-3-methyl benzofuran in phosphorus oxychloride and the anhydrous DMF; successively synthesizing 2-aryl-3-methanol benzofuran with sodium borohydride in methanol; finally firstly synthesizing methanesulfonate with methanesulfonyl chloride in dichloromethane, then synthesizing 2-aryl-3-methyl benzofuran-benzimidazole with benzimidazole or 5,6-dimethyl benzimidazole in methylbenzene and on the basis, synthesizing the 2-aryl-3-methyl benzofuran-benzimidazole salt compound with brominated alkanes in methylbenzene. The compound shows better antitumor cytotoxic activity.
Owner:YUNNAN MINZU UNIV
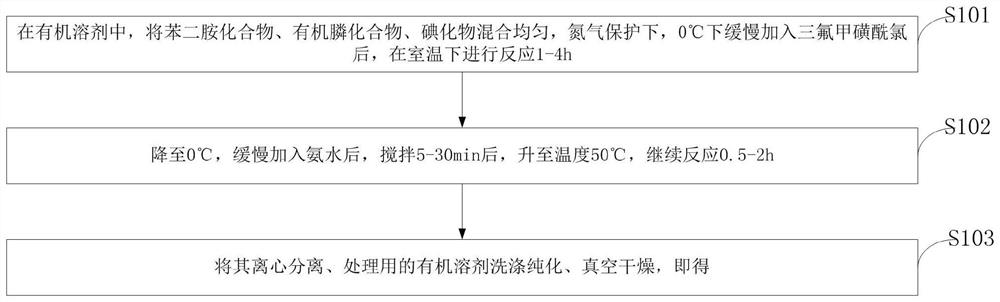
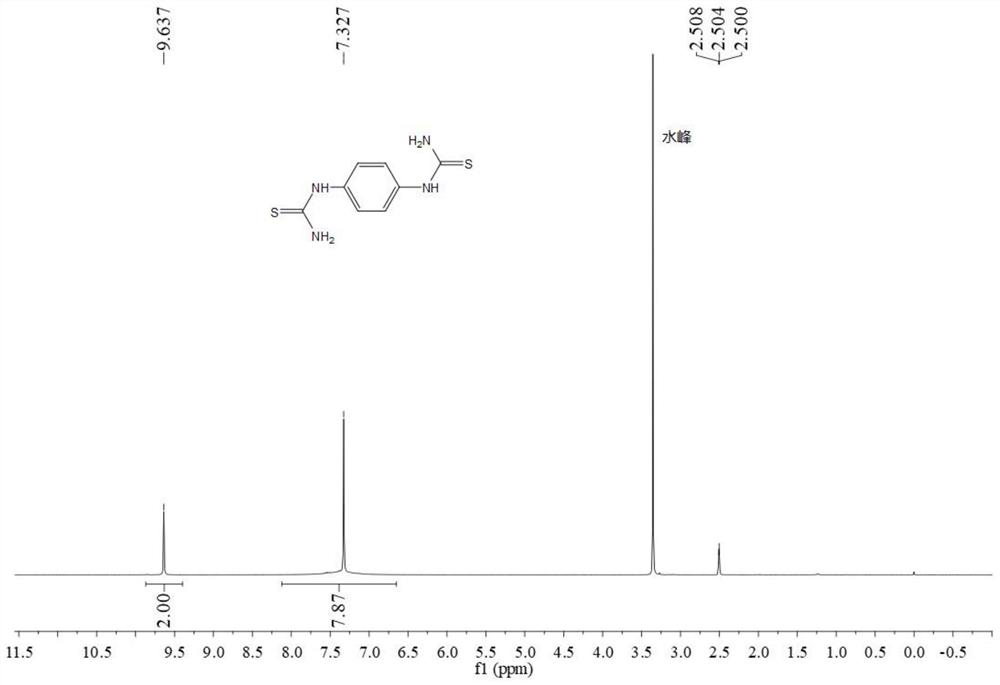
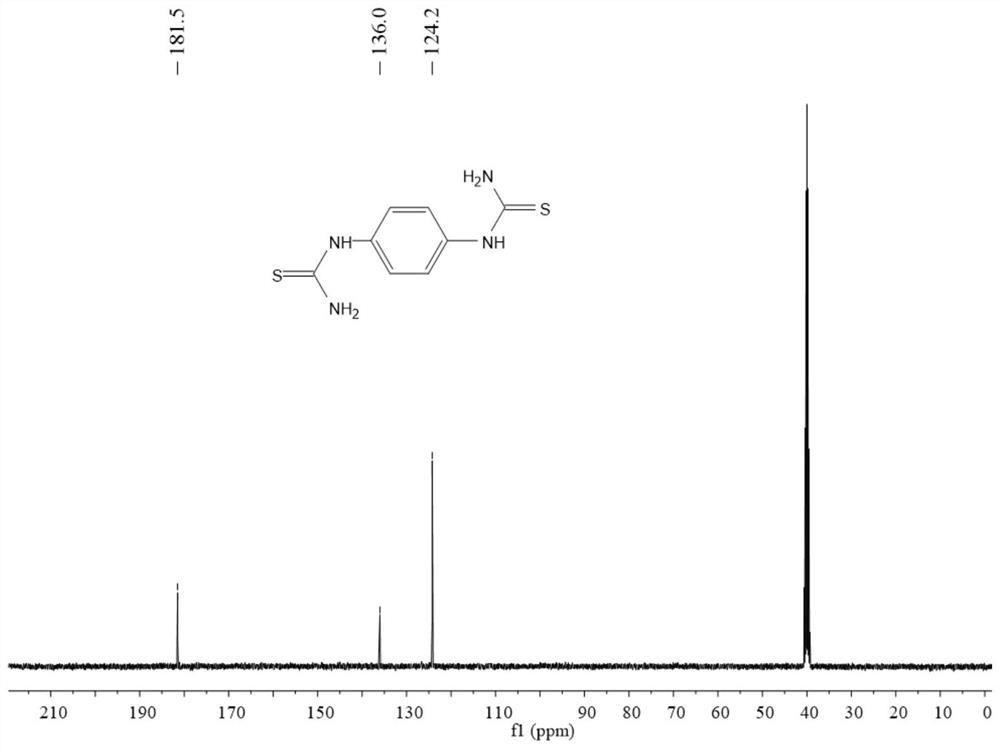
Preparation method of phenyl dithiourea compound
PendingCN114644582AIncrease structural diversityGood application effectOrganic chemistryOrganic solventIodide
The invention belongs to the technical field of COF monomer synthesis, and discloses a preparation method of a phenyl dithiourea compound. The preparation method comprises the following steps: uniformly mixing a phenylenediamine compound, an organic phosphine compound and iodide in an organic solvent, slowly adding trifluoromethanesulfonyl chloride at 0 DEG C under the protection of nitrogen, and reacting at room temperature for 1-4 hours; cooling to 0 DEG C, slowly adding ammonia water, stirring for 5-30 minutes, heating to 50 DEG C, and continuously reacting for 0.5-2 hours; and carrying out centrifugal separation, washing and purifying with an organic solvent for treatment, and carrying out vacuum drying to obtain the phenyl dithiourea compound. According to the preparation method of the phenyl dithiourea compound, one-pot reaction preparation is adopted, the process is mild and easy to control, the purity and yield of the product are high, the substrate universality is high, aftertreatment is simple and rapid, and the product can be prepared on an amplification scale. Due to the advantages, the preparation cost of the phenyl dithiourea compound is remarkably reduced, and the phenyl dithiourea compound has good industrial application value.
Owner:ZHONGYUAN ENGINEERING COLLEGE
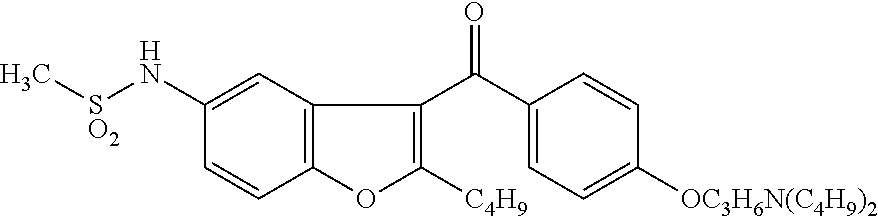
Preparation method of dronedarone hydrochloride and intermediate of dronedarone hydrochloride
InactiveCN102675267BInhibit side effectsImprove responseOrganic compound preparationAmino-hyroxy compound preparationPtru catalystAcyl group
The invention discloses a preparation method of an intermediate of dronedarone hydrochloride, which comprises the step of reacting 2-butyl-5-amino benzofuran hydrochloride serving as a starting material with methylsulfonyl chloride in the presence of a basic catalyst and an organic solvent to produce the intermediate of dronedarone hydrochloride, wherein the intermediate is 2-butyl-5-((N, N-dimethyl sulfonyl) amido) benzofuran. The invention also discloses a method for preparing dronedarone hydrochloride by using the intermediate. The preparation of the intermediate is simple and easy to operate; amino is protected by methylsulfonyl, so that the side reaction possibly generated in the reaction for preparing the dronedarone hydrochloride at the later period can be avoided, and the difficulty in purification at the later period can be simplified; the dronedarone hydrochloride yield is high; the problems in the prior art that the preparation of dronedarone hydrochloride is complicated with high cost, the intermediate is difficult to purify and aftertreatment is complicated can be solved; and the preparation difficulty of dronedarone hydrochloride is greatly reduced.
Owner:山东富创医药科技有限公司
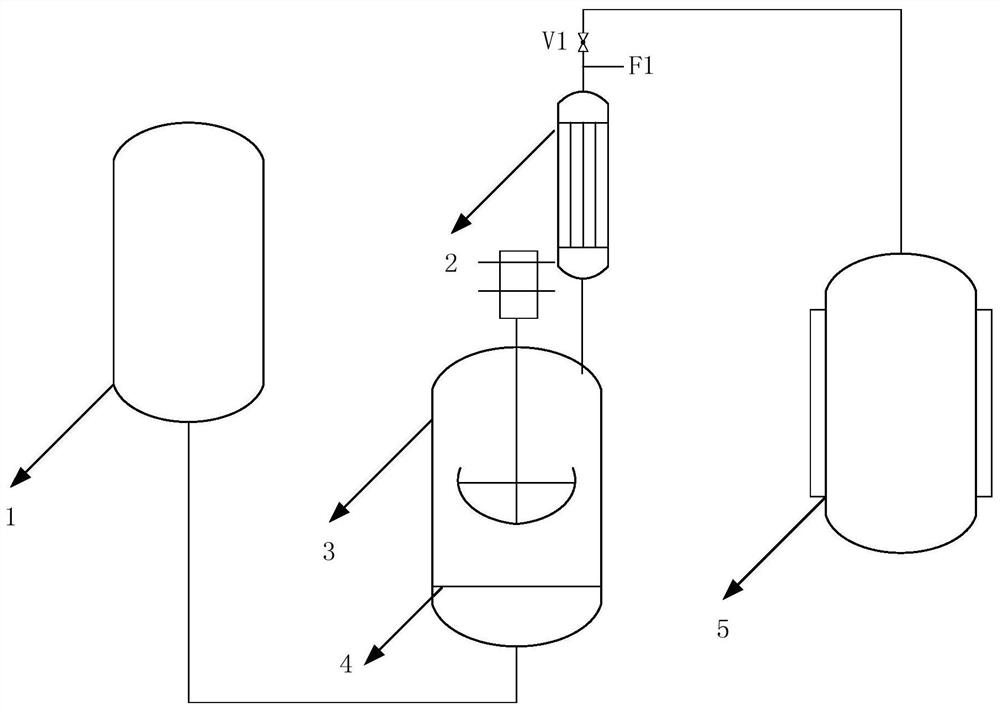
Device and method for preparing trifluoromethanesulfonyl fluoride
ActiveCN112724047ASimple processImprove securityProcess control/regulationOrganic compound preparationCyclohexanoneHydrogen fluoride
The invention relates to a device and a method for preparing trifluoromethanesulfonyl fluoride, and belongs to the technical field of trifluoromethanesulfonyl fluoride preparation. The device comprises a trifluoromethanesulfonyl chloride storage tank, a reactor, a reflux heat exchanger and a trifluoromethanesulfonyl fluoride storage tank; the device is few in equipment, the technological process is simplified, the safety is high, trifluoromethanesulfonyl fluoride with high purity can be collected while reaction is conducted, the equipment utilization efficiency is greatly improved, and the cost is effectively reduced; according to the method, trifluoromethanesulfonyl chloride and potassium fluoride are used as raw materials, cyclohexanone is used as a solvent, and an unreacted excessive fluorinating agent and the solvent are convenient to recover; according to the method, trifluoromethanesulfonyl fluoride with the purity of 99.5 vol% or above can be collected through simple reflux after reaction at a high temperature, the product purity is high, hydrogen fluoride and other light component impurities are avoided, and the method is suitable for industrial production.
Owner:PERIC SPECIAL GASES CO LTD
Reaction process and device for continuously synthesizing 18-crown ether-6
ActiveCN114031602AQuick responseShort reaction timeOrganic chemistryTransportation and packagingMethanesulfonyl chlorideBenzenesulfinic acid
The invention discloses a reaction process and device for continuously synthesizing 18-crown ether-6, and particularly relates to the field of crown ether. The reaction process comprises the steps: step 1, generating corresponding methyl benzene sulfonic acid dihydric alcohol ester or methanesulfonic acid dihydric alcohol ester on site through dihydric alcohol I and paratoluensulfonyl chloride or methanesulfonyl chloride; and step 2, directly carrying out cyclization reaction on the intermediate without separation and purification and corresponding dihydric alcohol II under the action of a potassium ion template to synthesize a target product. The problems of high intermittent operation energy consumption and low yield of an existing synthesis process or the need of additional synthesis due to difficulty in obtaining of intermediates in some processes are mainly solved. According to the invention, the continuous micro-channel process can be adopted to replace an existing intermittent stirring mode, so that the cost can be substantially reduced, the energy can be saved, the yield can be improved, and the rapid amplification can be achieved so as to be suitable for industrial production.
Owner:润峙之微流体科技(江苏)有限公司
Process for preparing levomilnacipran hcl
InactiveUS20140343322A1High purityHigh chiral purityOrganic compound preparationCarboxylic acid amides preparationTolueneAqueous solution
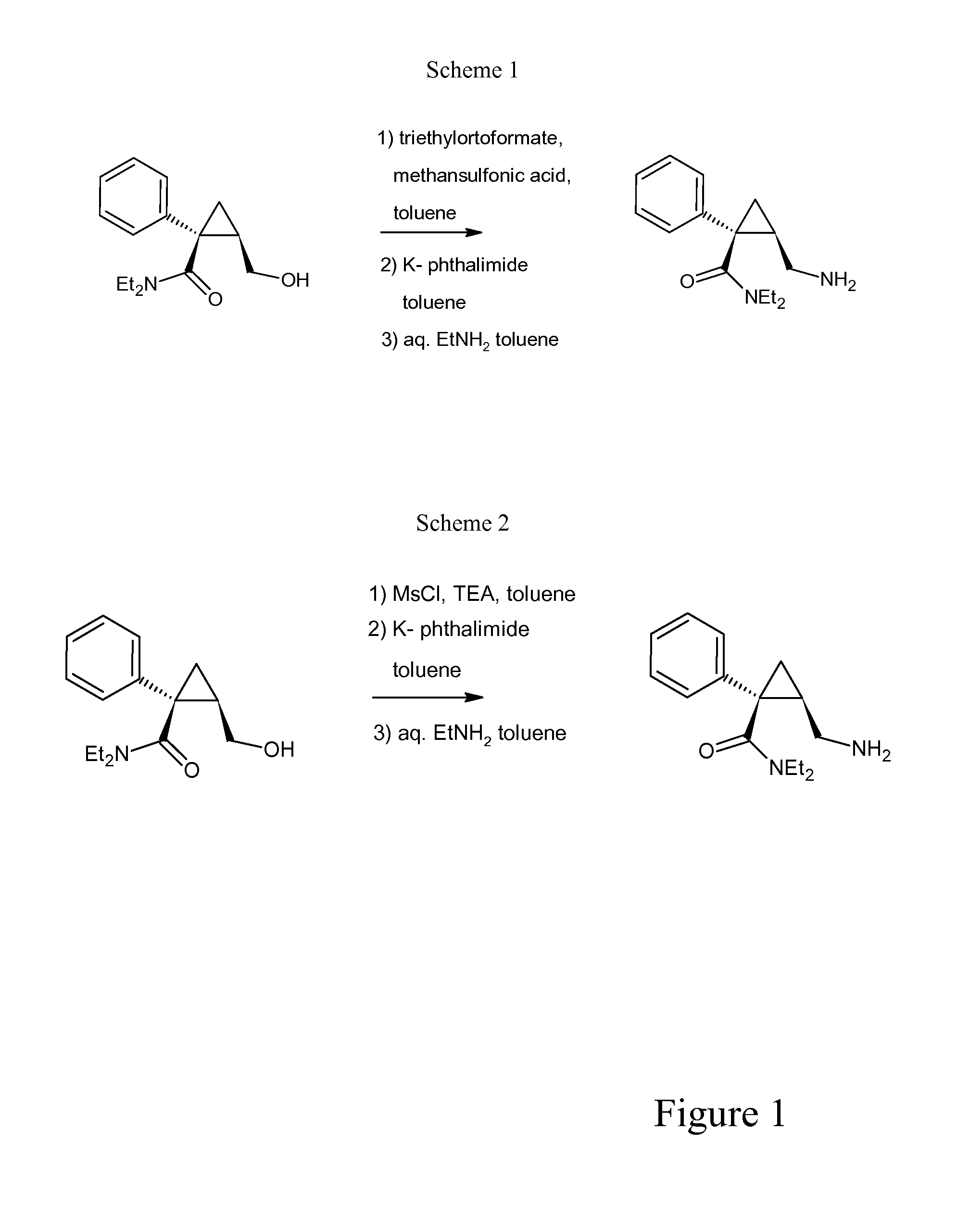
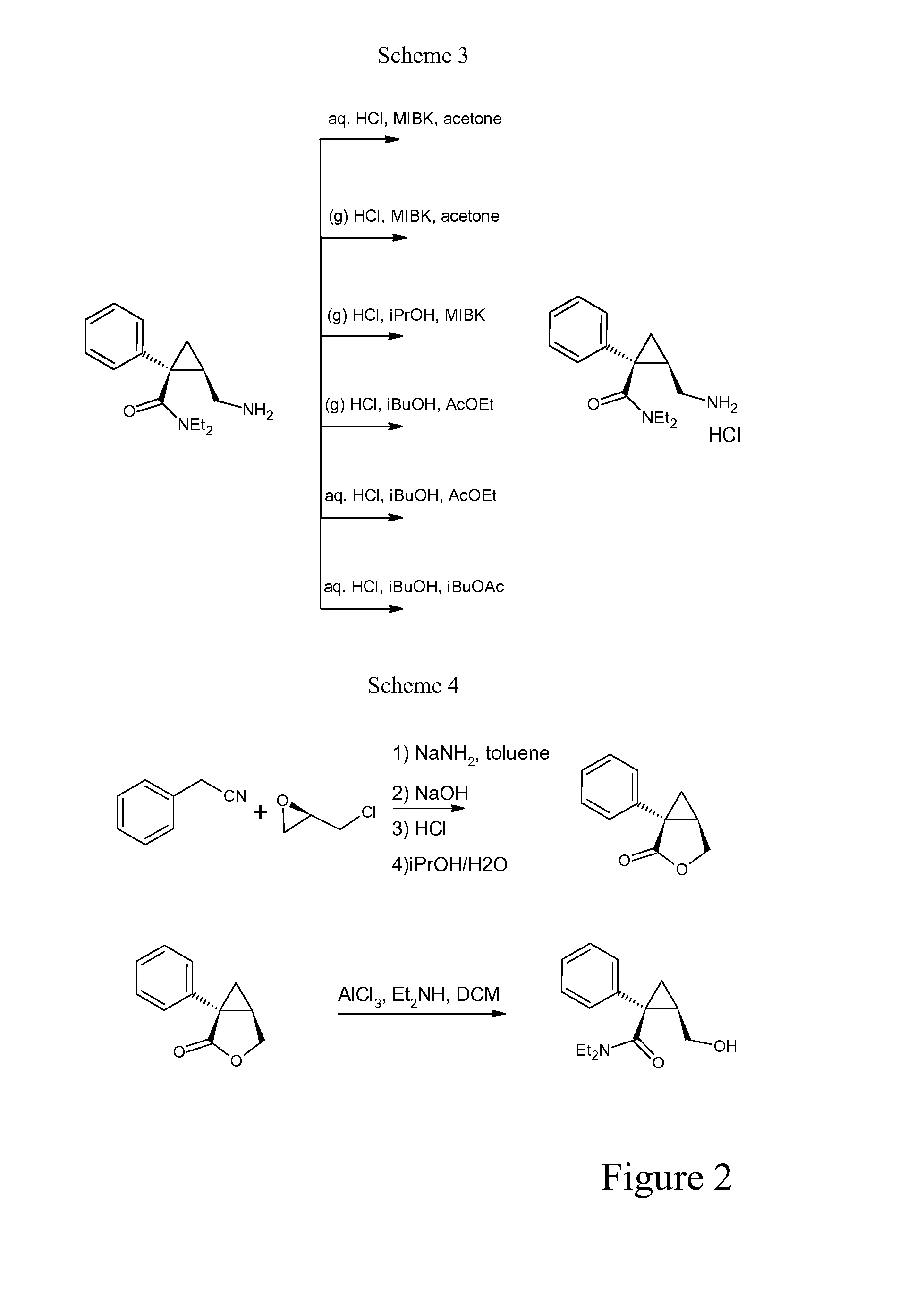
The invention relates to one-pot process for preparing (1S,2R)-1-phenyl-1-(N,N-diethylaminocarbonyl)-2-aminomethylcyclopropane of formula (I) comprising the step of reacting (1S,2R)-N,N-diethyl-2-(hydroxymethyl)-1-phenylcyclopropanecarboxamide successively with the following reactants 1) triethyl orthoformate and methanesulfonic acid or triethylamine and methanesulfonyl chloride, 2) a phthalimidating agent, 3) aqueous EtNH2, wherein the reaction is carried out in toluene. In another aspect the invention concerns a process for preparing (1S,2R)-N,N-diethyl-2-(hydroxymethyl)-1-phenylcyclopropanecarboxamide trough a step of crystallization of (1S,5R)-1-phenyl-3-oxabicyclo[3.1.0]hexan-2-one.
Owner:COSMA SPA
Method for preparing mesosulfuron-methyl by one-pot method
The invention discloses a method for preparing mesosulfuron-methyl by a one-pot method, which comprises the following steps: carrying out first-step methylsulfonylation reaction on 5-aminomethyl-2-methoxycarbonyl benzene sulfonamide and methylsulfonyl chloride in an organic solvent in the presence of 1, 8-diazabicyclo undec-7-ene; and directly adding 4, 6-dimethoxy-2-phenoxy carbonyl aminopyrimidine into an untreated system after the first-step methylsulfonylation reaction, and carrying out a second-step condensation reaction. According to the method, DBU and acetonitrile are respectively adopted as an acid-binding agent and a solvent in methylsulfonylation and are carried out at a relatively low temperature, so that an intermediate does not need to be separated by acid washing and water washing after methylsulfonylation, instead, the 4,6-dimethoxy-2-phenoxy carbonyl aminopyrimidine is directly added to prepare mesosulfuron-methyl; therefore, the separation of the 5-methylsulfonylamino methyl-2-methoxycarbonyl benzene sulfonamide is avoided, the operation is simple and convenient, and the purity and the yield of the product are relatively high.
Owner:江苏省农用激素工程技术研究中心有限公司 +1
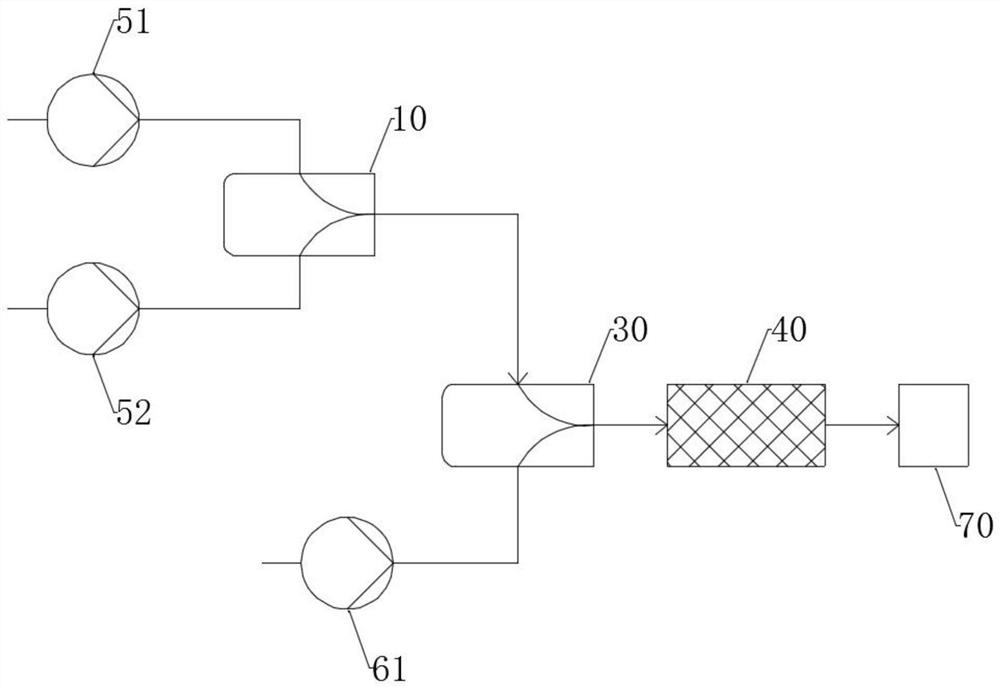
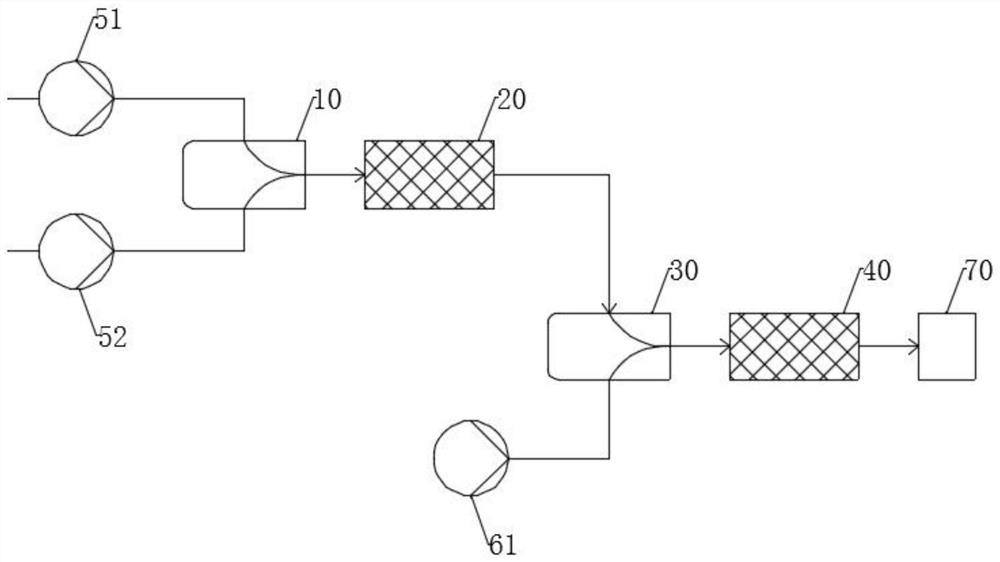
Purification device and method of bis (trifluoromethanesulfonyl) imine
ActiveCN114470826AEasy to moveReduce distillateOrganic chemistryOrganic compound preparationImideDiimine
The invention provides a bis (trifluoromethanesulfonyl) imine purification device which comprises a mixing kettle, the bottom of the mixing kettle is connected with a rectifying tower, the top of the rectifying tower is sequentially connected with a trifluoromethanesulfonyl chloride condenser and a bis-imine condenser, the bottom of the trifluoromethanesulfonyl chloride condenser is connected with a trifluoromethanesulfonyl chloride storage tank, and the bottom of the bis-imine condenser is connected with a bis-imine storage tank. The bottom of the diimine condenser is connected with a diimine storage tank; the invention also provides a method for purifying bis (trifluoromethanesulfonyl) imine by adopting the device, which comprises the following steps: adding crude bis (trifluoromethanesulfonyl) imine and high-purity water into the mixing kettle, stirring, then adding trifluoromethanesulfonyl chloride, introducing the uniformly mixed material into the rectifying tower, distilling off trifluoromethanesulfonyl chloride and water, then adjusting the parameters of the rectifying tower, and finally purifying the bis (trifluoromethanesulfonyl) imine. The high-quality bis (trifluoromethanesulfonyl) imine is obtained. According to the method, purification of bis (trifluoromethanesulfonyl) imine is completed by controlling the adding proportion of high-purity water, bis (trifluoromethanesulfonyl) imine and trifluoromethanesulfonyl chloride and rectification conditions.
Owner:PERIC SPECIAL GASES CO LTD
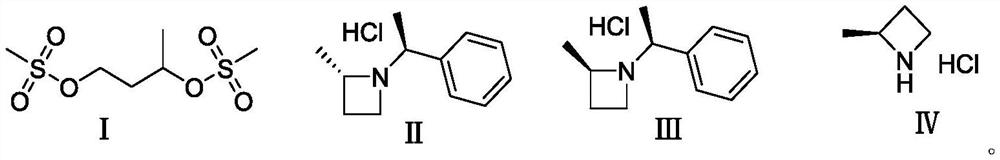
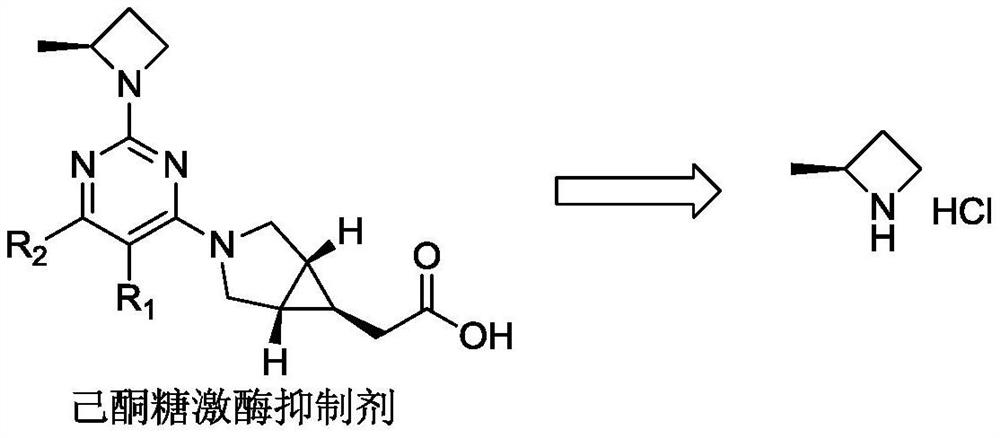
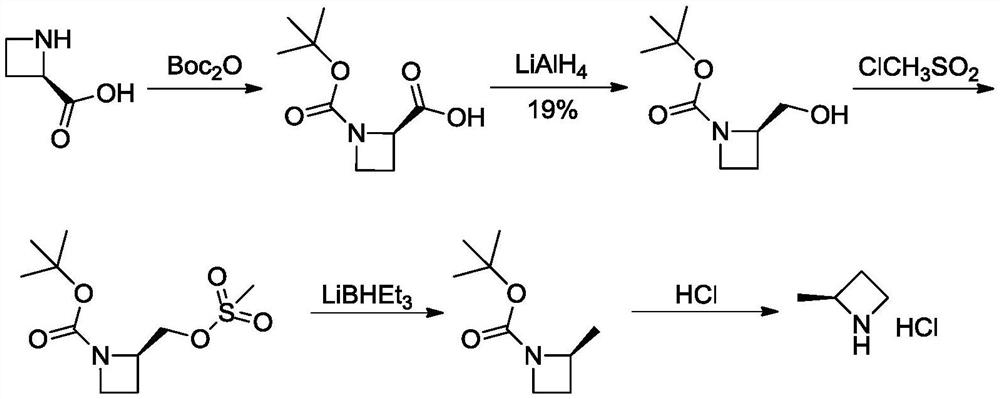
Preparation method of (S)-2-methyl azetidine hydrochloride
PendingCN114315494ALow priceThe reaction is easy to operateOptically-active compound separationOrganic racemisationOrganic synthesisCombinatorial chemistry
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of an intermediate (S)-2-methyl azetidine hydrochloride of a hexanketokinase inhibitor, which comprises the following steps: esterifying 1, 3-butanediol and methanesulfonyl chloride to obtain a compound shown as a formula (I), cyclizing the compound shown as the formula (I) and S-phenylethylamine to obtain a mixture of a compound shown as a formula (II) and a compound shown as a formula (III), and carrying out recrystallization to obtain the (S)-2-methyl azetidine hydrochloride of the hexanketokinase inhibitor. Refining and splitting the mixture to obtain a compound shown as a formula (II), and deprotecting the compound shown as the formula (II) to obtain a compound shown as a formula (IV). The invention provides a simple and cost-reducing industrial production route for the intermediate of the hexanketokinase inhibitor, and has the advantages of low price of initial raw materials, simple and convenient reaction, high resolution yield, low cost and capability of realizing stable industrial production and preparation.
Owner:苏州楚凯药业有限公司
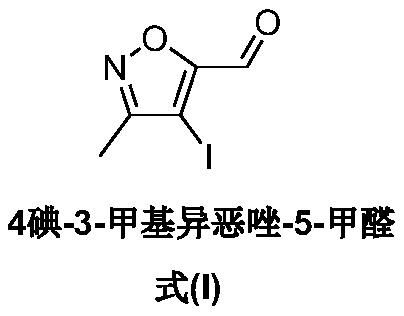
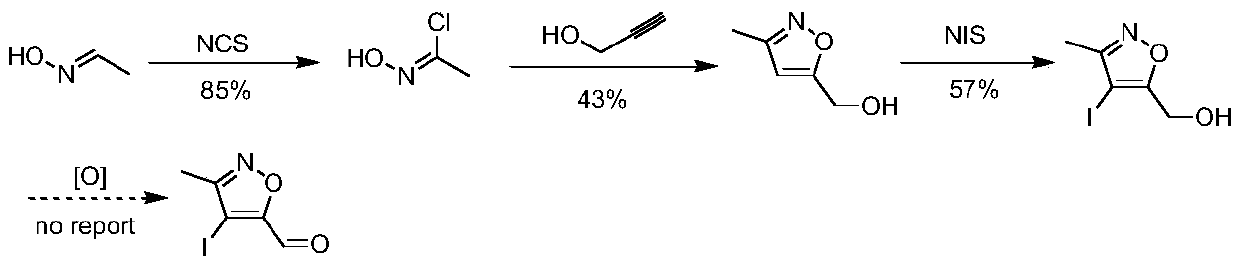
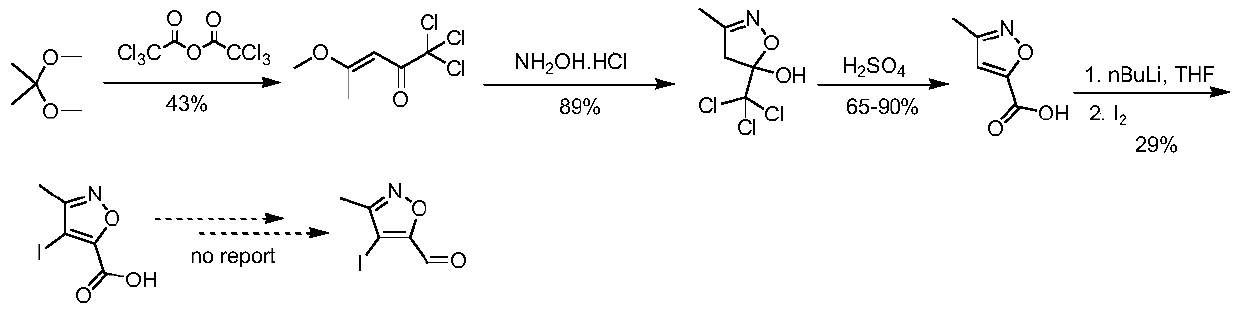
A kind of synthetic method of 4-iodo-3-methylisoxazole-5-carbaldehyde
The invention belongs to the technical field of organic synthesis and provides a synthesizing method of 4-iodo-methylisoxazole-5-formaldehyde. The synthesizing method of the 4-iodo-methylisoxazole-5-formaldehyde comprises (1) condensation reaction including condensing the raw material of acetone oxime with 2, 2-ethyl diethoxyacetate to obtain 5-(diethoxymethyl)-3-methyl-4, 5-dihydroisoxazole-5-alcohol, (2) acylation reaction including aromatizing the 5-(diethoxymethyl)-3-methyl-4, 5-dihydroisoxazole-5-alcohol through methylsulfonyl chloride to obtain 5-(diethoxymethyl)-3-methylisoxazole, (3) iodination reaction including iodinating the 5-(diethoxymethyl)-3-methylisoxazole in acetonitrile through iodosuccinimide to obtain the 4-iodo-methylisoxazole-5-formaldehyde. The preparation method ofthe 4-iodo-methylisoxazole-5-formaldehyde is short in reaction path, low in costs of raw materials, simple in operation and applicable large-scale industrial production.
Owner:成都道合尔医药技术有限公司
Process of synthesizing (2S-trans)-3-methyl-4-oxo-1-azacyclo butyl sulfonic acid
The present invention relates to process of synthesizing (2S-trans)-3-amino-2-methyl-4-oxo-1-azacyclo butyl sulfonic acid. The process includes the following steps: 1. reaction of L-threonine and matter to protect its amino group; 2 condensation of L-threonine with protected amino group and aminosulfonic acid to obtain threonyl aminosulfonic acid with protected amino group; 3. reaction of threonyl aminosulfonic acid with protected amino group and methylsulfnyl chloride to protect hydroxyl radicap in threonine segment; 4. cyclization reaction to form protected quaternary ammonium salt of azacyclo butyl sulfonic acid; and 5. deprotection to obtain (2S-trans)-3-amino-2-methyl-4-oxo-1-azacyclo butyl sulfonic acid.
Owner:CHINA RESOURCES SAIKE PHARMA
A ratiometric two-photon fluorescent probe and its preparation method and application
ActiveCN111875560BDeep penetrationAvoid damageOrganic chemistryFluorescence/phosphorescenceFluoProbesSuperoxide
The invention belongs to the technical field of synthesis and fluorescence detection, and discloses a ratio-type two-photon fluorescent probe and its preparation method and application. The preparation method of the fluorescent probe is: reacting hydroxytetraphenylethylene with hexamethylenetetramine to obtain Compound 1, compound 1 reacts with o-aminothiophenol to obtain compound 2, adding trifluoromethanesulfonyl chloride to compound 2 to generate the final product, which is the ratio-type two-photon fluorescent probe TPE-BT-TF; the fluorescent probe is applied For the detection of superoxide anion, the concentration of superoxide anion can be determined, and the selectivity is strong; the fluorescent probe has two-photon excitation characteristics, and can use two-photon confocal microscopy to perform ratiometric double-photon detection of superoxide anion in samples such as cells. Photon fluorescence imaging detection, the imaging effect is good.
Owner:SHANDONG NORMAL UNIV
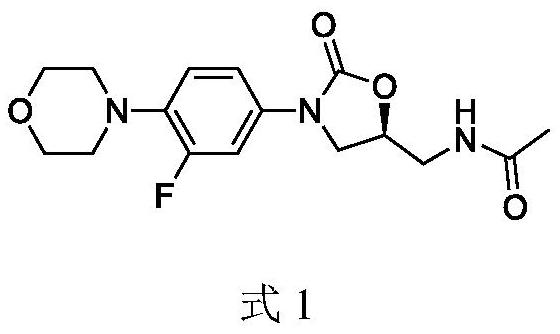
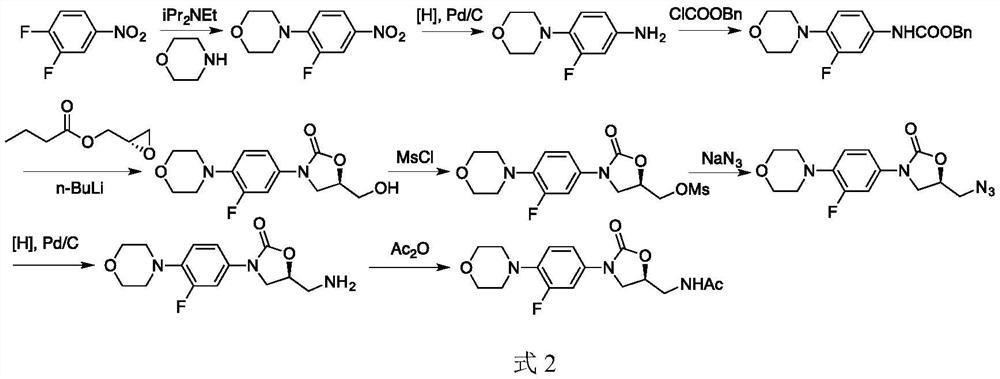
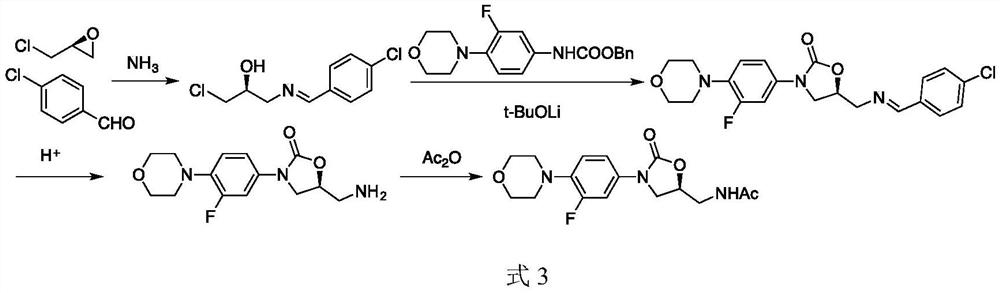
A kind of preparation method of linezolid intermediate
The invention discloses a preparation method of a linezolid intermediate. Using 3-fluoro-4-(4-morpholino)-aniline and 2-((S)-3-chloro-2-hydroxypropyl) isoindoline-1,3-dione as main raw materials, In an amide dipolar solvent, at a temperature of 90-150°C, a substitution reaction occurs to obtain an important intermediate N-(3-phthalimido-2-(S)-hydroxypropyl base)-3-fluoro-4-(morpholinyl)aniline. The preparation steps of the intermediate are simple, and the preparation process does not use dangerous reagents such as butyllithium, sodium azide, chloroformate, methanesulfonyl chloride, etc. in the conventional process, which are flammable, explosive, and highly toxic, and have high safety. ; The main raw materials 3‑fluoro‑4‑(4‑morpholinyl)‑aniline and 2‑((S)‑3‑chloro‑2‑hydroxypropyl) isoindoline‑1,3‑dione are stable sources , low cost, and the used amide dipole solvent is easy to recycle, which can reduce environmental pollution.
Owner:泰州申合泰尔生物医药有限公司
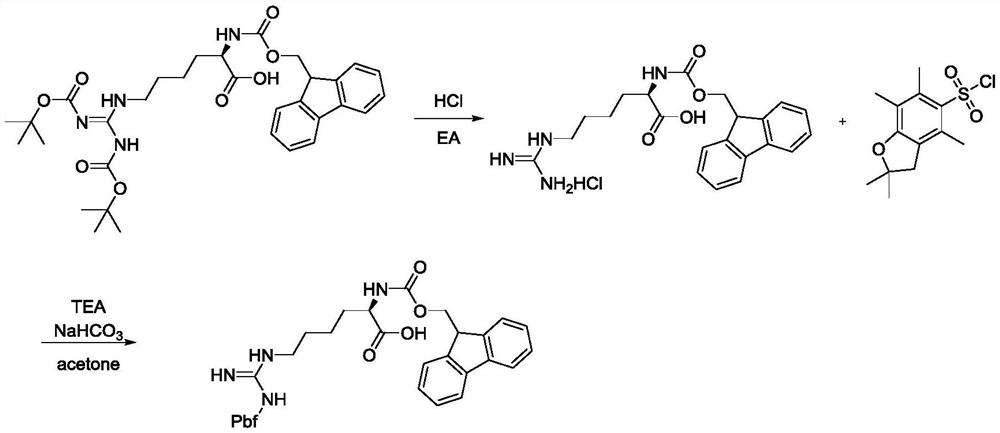
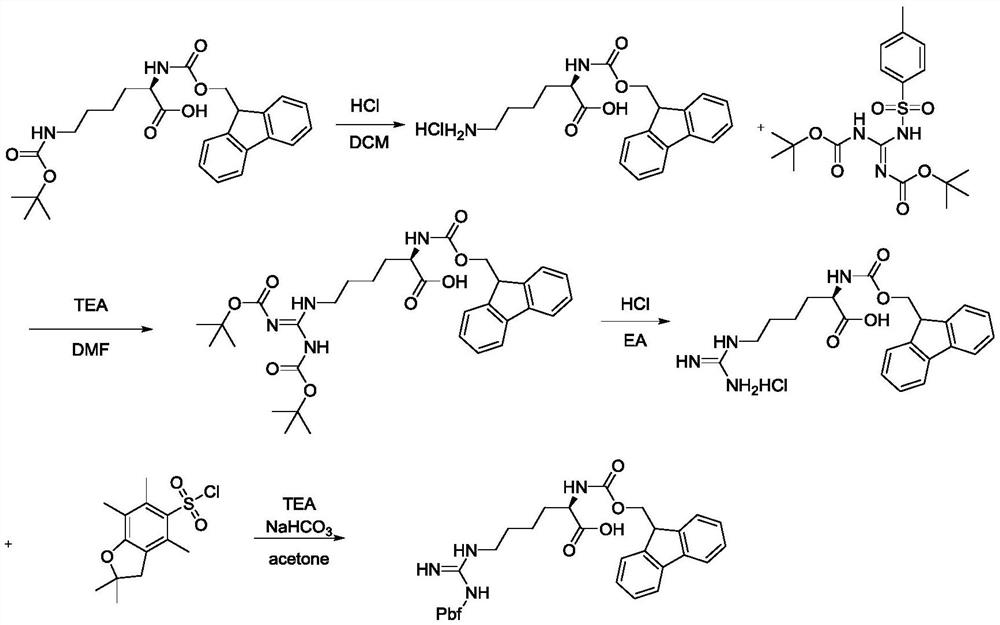
Preparation method of eptifibatide key raw material L-higher arginine
InactiveCN112375055AEasy to synthesizeLow costCarbamic acid derivatives preparationOrganic compound preparationTrifluoromethanesulfonic anhydrideArginine
The invention discloses a preparation method of an eptifibatide key raw material L-higher arginine, and belongs to the field of synthesis of medical intermediates. The preparation method comprises thefollowing steps: reacting 1,3-bis(tert-butyloxycarbonyl)guanidine with p-toluenesulfonyl chloride to obtain 1,3-bis-Boc-2-(p-toluenesulfonyl)guanidine, then reacting the 1,3-bis-Boc-2-(p-toluenesulfonyl)guanidine with N-fluorenylmethoxycarbonyl-N'-tert-butyloxycarbonyl-L-lysine Fmoc-hArg(Boc2)-OH, removing Boc protection, and feeding Pbf-Cl to obtain N-(9-fluorenylmethoxycarbonyl)-2,2,4,6,7-pentamethyl-2H-benzofuran-5-sulfonyl-L-arginine. According to the invention, a p-toluenesulfonyl polypeptide guanidinylating agent is adopted in the route, so that ultralow-temperature reaction and the useof trifluoromethanesulfonyl chloride or trifluoromethanesulfonic anhydride with high corrosivity are avoided, and a simple and efficient way is provided for the synthesis of the intermediate.
Owner:宁夏蓝博思化学技术有限公司
Method for mildly preparing 2-azaspiro [3.3] heptane hydrochloride
InactiveCN112920103AImprove reducibilityHigh purityOrganic chemistryBulk chemical productionNitrobenzeneAcyl group
The invention relates to a method for mildly preparing 2-azaspiro [3.3] heptane hydrochloride, and solves the technical problem that in historical literatures, harsh conditions of strong base sodium are needed. The synthesis method comprises the following steps: (1) reducing cyclobutane-1,1-dicarboxylic acid into dimethyl alcohol; (2) reacting the 1, 1-cyclobutane dimethyl carbinol with methanesulfonyl chloride to generate 1,1-cyclobutane dimethyl carbinol dimethyl sulfonate; (3) carrying out ring closing on the 1,1-cyclobutane dimethyl carbinol dimethyl sulfonate and the 2-nitrobenzene sulfonamide to generate 2-(2-nitrobenzenesulfonyl)-2-azaspiro [3.3] heptane; (4) enabling the 2-(2-nitrobenzenesulfonyl)-2-azaspiro [3.3] heptane to react with dodecanethiol under the action of DBU to remove the 2-nitrobenzenesulfonyl so as to generate 2-azaspiro [3.3] heptane; and (5) reacting the 2-azaspiro [3.3] heptane with BOC anhydride to generate Boc-2-azaspiro [3.3] heptane, and then performing treatment with hydrochloric acid to obtain the 2-azaspiro [3.3] heptane hydrochloride. The method is mild and easy to operate, and avoids violent conditions of removing amino protecting groups by strong base at high temperature.
Owner:KANGHUA SHANGHAI DRUG RES DEV CO LTD
Synthesis method of bisamide insecticide
The invention discloses a synthesis method of a bisamide insecticide, which relates to the technical field of organic synthesis. The synthesis method comprises the steps that a pyrazoline carboxylic acid compound shown in the general formula (III) and an anthranilamide compound shown in the general formula (II) serve as raw materials; methanesulfonyl chloride is used as an oxidizing agent and a condensing agent to synthesize the bisamide insecticide as shown in the general formula (I) in one step. According to the synthesis method, a pyrazoline carboxylic acid compound and an anthranilamide compound are used as raw materials, methylsulfonyl chloride is used as an oxidizing agent and a condensing agent, a coupling reaction and an oxidation reaction are carried out at the same time under the action of certain tertiary amine, in the coupling reaction process, pyrazoline can be epoxidized into a pyrazole ring at the same time, the bisamide insecticide with high purity and high yield is prepared by adopting the method, the traditional oxidation reaction carried out by adopting an oxidizing agent is successfully replaced, the reaction steps are simplified, the pollution to the environment is reduced, and the reaction safety is improved.
Owner:SHANDONG ACADEMY OF PESTICIDE SCI
Synthesis method of oseltamivir intermediate
PendingCN114685297AImprove securityHigh yieldOrganic compound preparationAmino-carboxyl compound preparationChemical compoundPharmaceutical drug
The invention discloses a synthesis method of an oseltamivir intermediate, which comprises the following steps of: carrying out addition on a compound 1 and diallylamine under the protection of acid, and then carrying out ring opening and tert-butyl nucleophilic addition to obtain the oseltamivir intermediate, and carrying out three-step reaction to obtain the oseltamivir intermediate. Compared with an existing industrial six-step reaction route, the reaction route is remarkably shortened, the yield is high, the overall yield exceeds 70%, and the production efficiency is improved. Meanwhile, according to the synthesis method, methylsulfonyl chloride cyclization is not needed, introduction of impurities with a genetic toxicity warning structure is avoided, and the medicine safety is improved. Moreover, the synthesis method does not use any azide, is high in safety, and is suitable for industrial large-scale production.
Owner:中润药业有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com
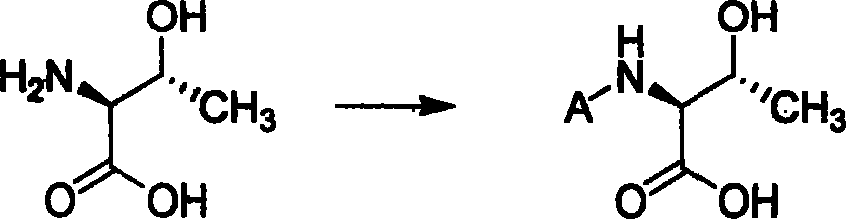
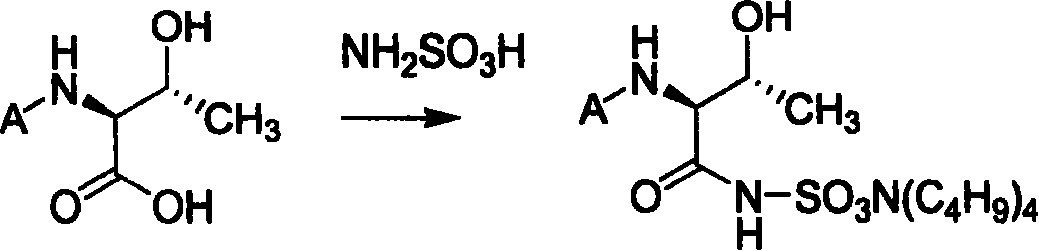
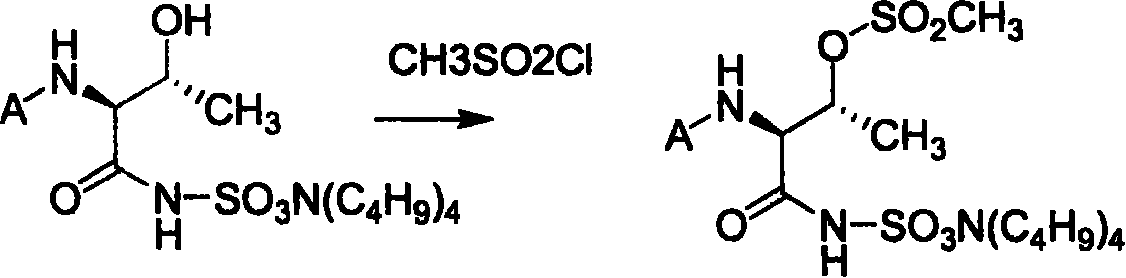
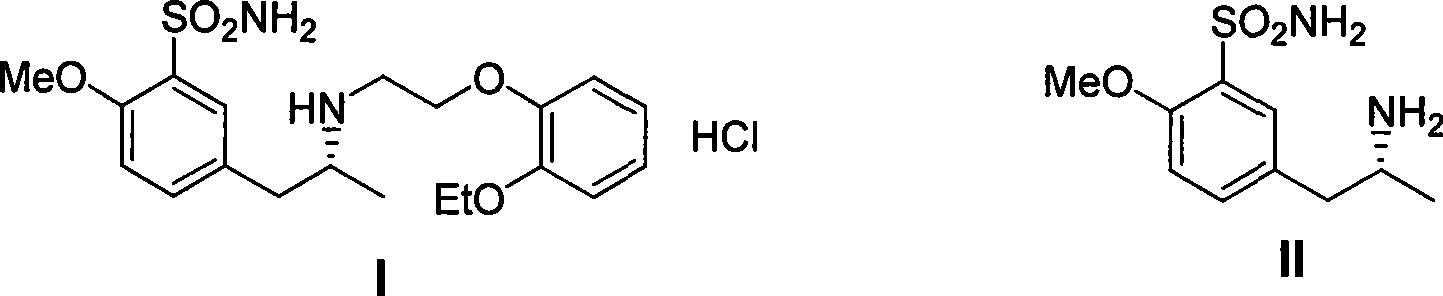

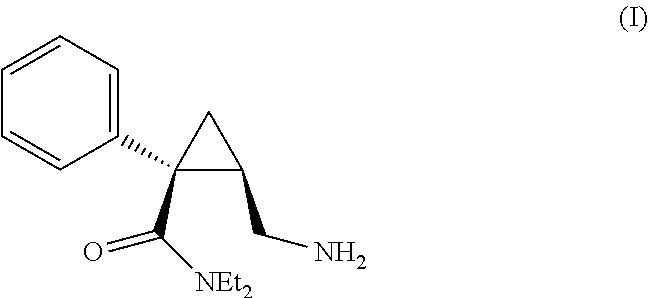


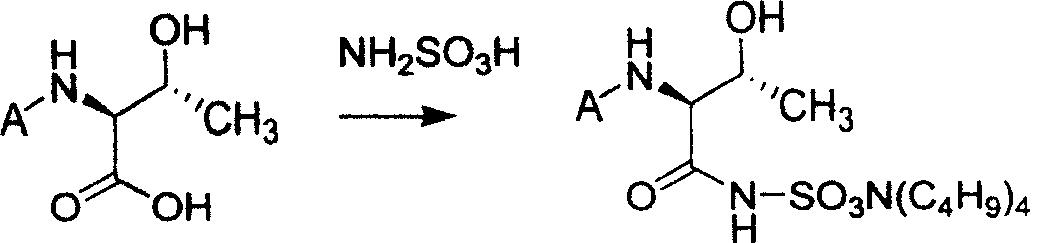
![Method for mildly preparing 2-azaspiro [3.3] heptane hydrochloride Method for mildly preparing 2-azaspiro [3.3] heptane hydrochloride](https://images-eureka.patsnap.com/patent_img/2eeff962-2fb2-4d70-8126-a50c10941319/954037DEST_PATH_IMAGE001.png)