Reaction process and device for continuously synthesizing 18-crown ether-6
A technique and technology of crown ethers, which are applied in the field of reaction techniques and devices for continuous synthesis of 18-crown ether-6, can solve the problems of high energy consumption, low yield, and difficult availability of intermediates in the intermittent operation of the synthesis process, and achieve production High efficiency, increased output, and labor-saving effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
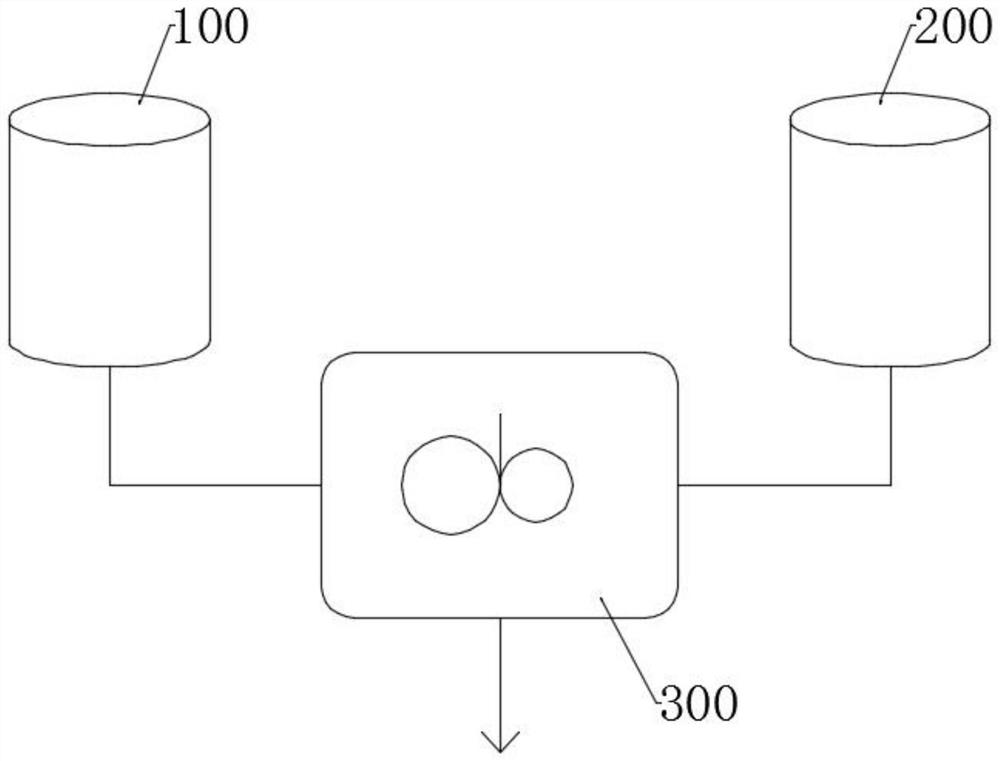
[0033] Such as image 3 Shown, at room temperature, with triethylene glycol, acid agent triethylamine molar ratio is 1:2 and solvent (the THF concentration of triethylene glycol substrate after mixing is 4 mol / liter) from feeding tank 100 and The addition tank 200 is added to the mixer 300 with electromagnetic or mechanical stirring and mixed to prepare the first premix;
[0034] In addition, at room temperature, p-toluenesulfonyl chloride (with the substrate triethylene glycol molar ratio of 2:1) and the solvent THF were mixed into a second premixture with a concentration of 8 mol / L;
[0035] With triethylene glycol, alkali potassium hydroxide molar ratio is 1:2 and solvent (the dioxane concentration of triethylene glycol substrate after mixing is 4 mol / liter) joins from feed tank 100 and feed tank 200 to following Mix in the mixer 300 of electromagnetic or mechanical agitation, make the 3rd premix;
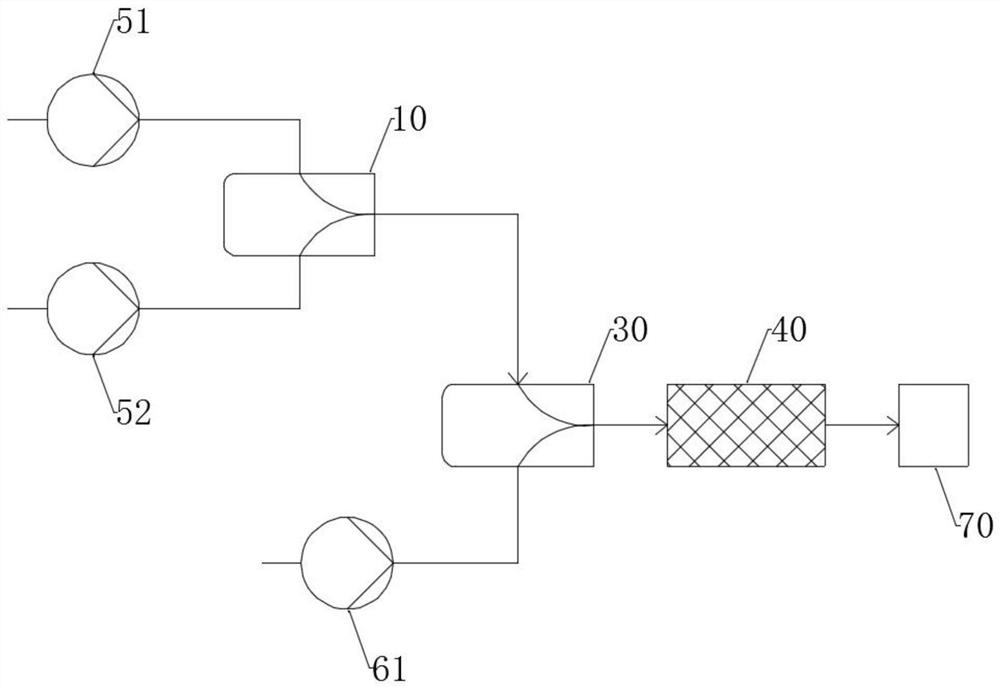
[0036] Such as figure 1 As shown, at room temperature, the first premix ...
Embodiment 2
[0038] The difference with embodiment 1 is:
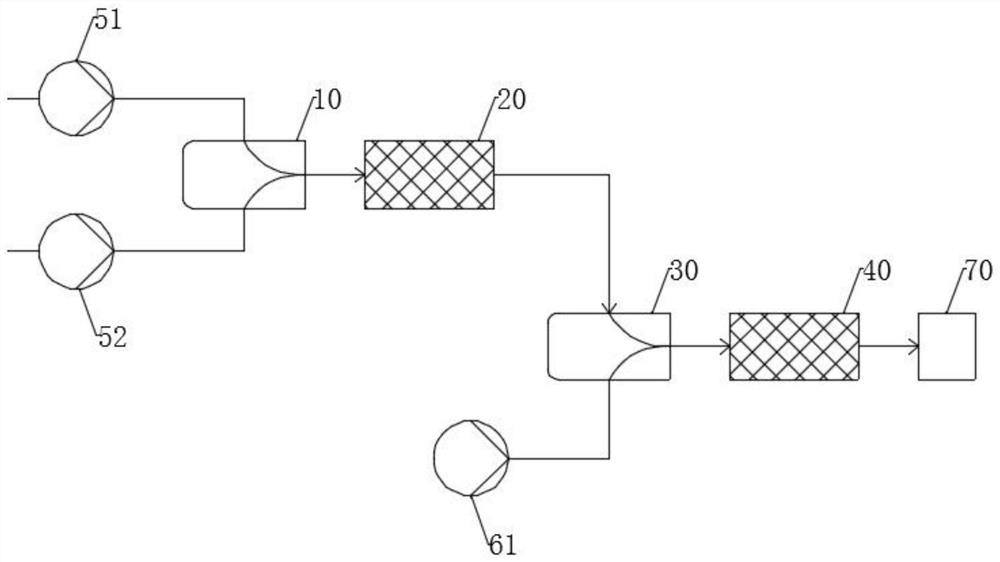
[0039] Such as figure 2 As shown, at room temperature, the first premix and the second premix are input to the micro-mixer 10 through the pump 51 and the pump 52 respectively, and the volume flows of the first premix and the second premix are respectively 10.0 mL / min and 10.0mL / min, the total volume flow rate is 20.0mL / min. The mixer 10 is an interdigitated micro-mixer with a channel size of 85 microns. Before entering the second micro-mixer 30, at first through the micro-reactor 20 to carry out sufficient esterification reaction site to generate the corresponding diol tosylate, then the mixture and the 3rd pre-mixture through the pump 61 in the micro-reactor 20 Mixing is carried out in the mixer 30 and subsequent serial etherification and cyclization reactions take place. The feed volume flow rate of each material remains the same, and the configuration of the mixer and the reactor is also unchanged. Reactor 20 is a flat-tube...
Embodiment 3
[0041] The difference with embodiment 2 is:
[0042] Such as image 3 As shown, at room temperature, the molar ratio of triethylene glycol and alkali potassium hydroxide is 1:2 and solvent (the dioxane concentration of the triethylene glycol substrate after mixing is 4 mol / liter), and a catalytic amount of 18-Crown ether-6 (molar ratio of 1:100 to the substrate diol) is added from the feed tank 100 and the feed tank 200 to the mixer 300 with electromagnetic or mechanical stirring and mixed to prepare the third pre-mixture;
[0043] Such as figure 2 As shown, the feed volume flow rate of each material remains the same, and the configuration of the mixer and the reactor is also unchanged. The crude reaction product was desalted, separated, extracted, solvent evaporated, and the crude product was distilled under reduced pressure to obtain 18-crown-6 with a yield of 76% and a total residence time of 7.5 min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com