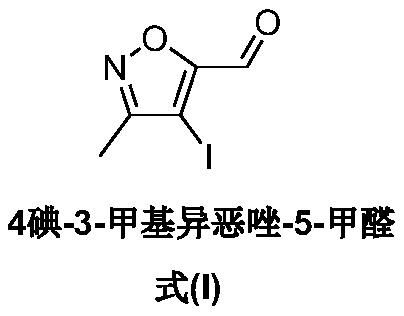
A kind of synthetic method of 4-iodo-3-methylisoxazole-5-carbaldehyde
A technique for synthesizing methylisoxazole and its synthesis method, which is applied in the field of synthesis of 4-iodo-3-methylisoxazole-5-carbaldehyde, can solve the problems of high raw material cost, low yield, long route, etc., and achieve The effect of low raw material cost, short reaction route and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1: Preparation of 5-(diethoxymethyl)-3-methyl-4,5-dihydroisoxazol-5-ol:
[0031]
[0032] Add 500 g (6.84 mol, 1.8 eq) of acetone oxime into 10 L of tetrahydrofuran and lower the temperature to 0-10°C. 6.84L (17.1mol, 4.5eq) of 2.5M n-butyllithium was added dropwise, and the internal temperature was controlled at 0-20°C. After the addition was complete, the mixture was stirred at -5-0°C for 0.5 hours. 670g (3.80mol, 1.0eq) of ethyl 2,2-diethoxyacetate was added dropwise, and the internal temperature was controlled at -5-0°C. After dropping, stir at 0-15°C for 3 hours. Add 15 L of 20% citric acid aqueous solution dropwise, adjust the pH=2, and control the inner temperature to less than 15°C. Stand still to separate the liquids, extract the aqueous phase with 3.0Lx2 ethyl acetate, combine the organic phases, and wash with 3L saturated brine. The organic phase was concentrated to dryness under reduced pressure, and was directly used in the next reaction to o...
Embodiment 2
[0034] Embodiment 2: the preparation of 5-(diethoxymethyl)-3-methylisoxazole:
[0035]
[0036] Add 300 g (1.48 mol, 1.0 eq) of 5-(diethoxymethyl)-3-methyl-4,5-dihydroisoxazol-5-ol 2 into 3 L of dichloroethane, and react The liquid drops to 0-10°C. Add 497g (3.85mol, 2.6eq) of diisopropylethylamine. 212g (1.85mol, 1.25eq) of methanesulfonyl chloride was added dropwise, and the internal temperature was controlled at 0-15°C. After dropping, react at 25°C for 12h. Add 1L of water, adjust the pH to 5-6 with 20% citric acid, and let stand to separate the liquid. The organic phase was washed with 1 L of saturated brine. The organic phase was concentrated to dryness under reduced pressure to obtain a crude product, which was distilled under reduced pressure to obtain 222 g of 5-(diethoxymethyl)-3-methylisoxazole 3 with a yield of 81%.
[0037] h 1 NMR (400 MHz, CDCl3): 6.21 (s, 1H), 5.63 (s, 1H), 3.66 (q, 4H), 2.33 (s, 3H), 1.27 (t, 6H).
Embodiment 3
[0038] Embodiment 3: Preparation of 4-iodo-3-methylisoxazole-5-carbaldehyde:
[0039]
[0040] Add 5-(diethoxymethyl)-3-methylisoxazole 3 300g (1.62mol, 1.0eq) into acetonitrile 1.5L, add iodosuccinimide 656g (2.92mol, 1.8eq) . 0.5 L of trifluoroacetic acid was added dropwise, and the temperature was raised to 65-75° C. to react for 12 hours. Concentrate under reduced pressure until no liquid flows out obviously, and cool down to room temperature. 1 L of 5% sodium carbonate solution was added, and the aqueous phase was extracted with 900 mL x 2 of ethyl acetate. The organic phases were combined and washed successively with 500 mL of 5% hydrosulfite solution and 500 mL of saturated brine. The organic phase was concentrated to dryness under reduced pressure to obtain a crude product, and the silica gel pad was shortened, rinsed with ethyl acetate / petroleum ether=1 / 7, and the filtrate was concentrated to dryness to obtain 4(4-iodo-3-methylisoxazole-5-carbaldehyde ) 261g, y...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com