Device and method for preparing trifluoromethanesulfonyl fluoride
A technology of trifluoromethanesulfonyl fluoride and trifluoromethanesulfonyl fluoride storage tank, applied in the field of trifluoromethanesulfonyl fluoride preparation, can solve the problems of hydrogen leakage and explosion, operation danger, influence on product purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
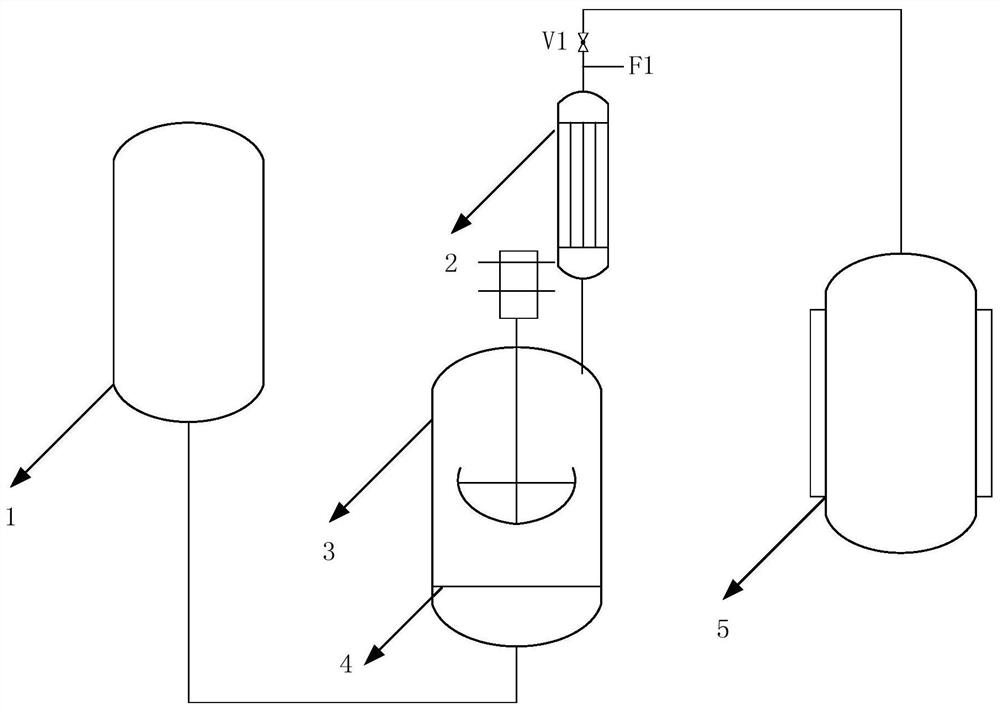
[0038] 10kg potassium fluoride and 16.25kg cyclohexanone (C 6 h 10 O), the heat transfer area is 1m 2 The temperature of the reflux heat exchanger 2 is controlled at -30°C to -25°C, and the reactor 3, the reflux heat exchanger 2, the trifluoromethanesulfonyl fluoride storage tank 5 and the pipelines between them are first vacuumed to 500Pa~1000Pa, then control the temperature of reactor 3 at 40℃~45℃, and then liquid trifluoromethanesulfonyl chloride (CF 3 SO 2 Cl) is pressed in from the bottom of the reactor 3 at a speed of 2.5kg / h, and the liquid trifluoromethanesulfonyl chloride enters the reactor 3 and vaporizes rapidly and passes through a gas disperser 4 with an aperture of 10 μm to react upwards with potassium fluoride, and the trifluoromethanesulfonyl chloride produced by the reaction reacts upwards with potassium fluoride. The fluoromethanesulfonyl fluoride passes through the reflux heat exchanger 2 and is collected in the trifluoromethanesulfonyl fluoride storage t...
Embodiment 2
[0041] In the 50L reactor 3, 10kg potassium fluoride and 16.7kg cyclohexanone are pre-loaded, and the heat exchange area is 1m 2 The temperature of the reflux heat exchanger 2 is controlled at -15°C to -10°C. First, the reactor 3, the reflux heat exchanger 2, the trifluoromethanesulfonyl fluoride storage tank 5 and the pipelines between them are vacuumized to 500Pa~1000Pa, then control the temperature of reactor 3 at 55℃~60℃, then press liquid trifluoromethanesulfonyl chloride from the bottom of reactor 3 at a speed of 4kg / h, and liquid trifluoromethanesulfonyl chloride enters reactor 3 Afterwards, it is quickly vaporized and reacts upward with potassium fluoride through a gas disperser 4 with a pore size of 30 μm, and the trifluoromethanesulfonyl fluoride produced by the reaction passes through a reflux heat exchanger 2 and is collected at a temperature of -20°C to -18°C. In methanesulfonyl fluoride storage tank 5.
[0042] In this embodiment, a total of 16.7 kg of trifluoro...
Embodiment 3
[0044] In the 50L reactor 3, 10kg potassium fluoride and 16kg cyclohexanone are pre-loaded, and the heat exchange area is 1m 2 The temperature of the reflux heat exchanger 2 is controlled at -20°C to -15°C, and the reactor 3, the reflux heat exchanger 2, the trifluoromethanesulfonyl fluoride storage tank 5 and the pipelines between them are first vacuumed to 500Pa~1000Pa, then control the temperature of reactor 3 at 55℃~60℃, then press liquid trifluoromethanesulfonyl chloride from the bottom of reactor 3 at a speed of 3.5kg / h, and liquid trifluoromethanesulfonyl chloride enters the reactor After 3, it is quickly vaporized and reacts upward with potassium fluoride through a gas disperser 4 with a pore size of 20 μm, and the trifluoromethanesulfonyl fluoride produced by the reaction passes through a reflux heat exchanger 2 and is collected in a trifluoromethanesulfonyl fluoride at a temperature of -10°C to -8°C. In the storage tank 5 of fluoromethanesulfonyl fluoride.
[0045] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com