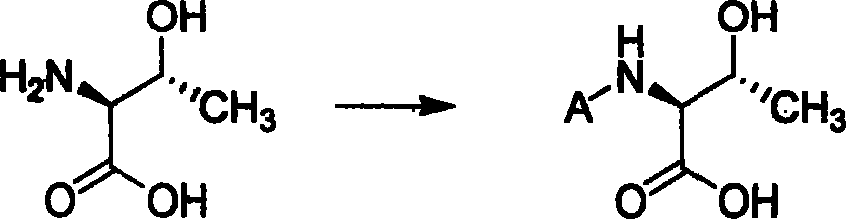
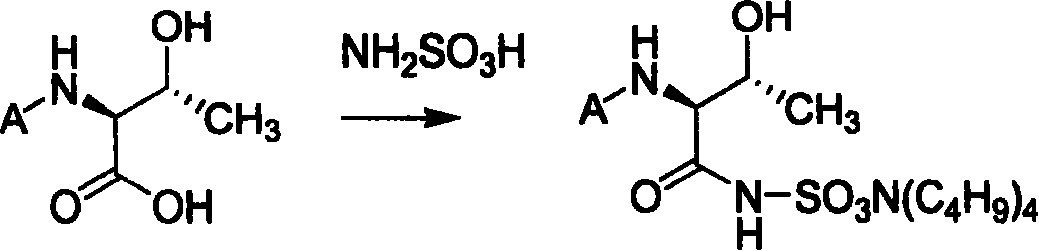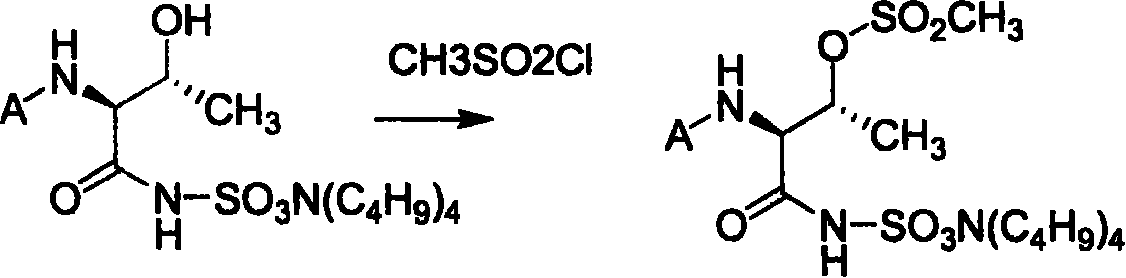Process of synthesizing (2S-trans)-3-methyl-4-oxo-1-azacyclo butyl sulfonic acid
A technology of azetidine sulfonic acid and sulfamic acid, which is applied in the direction of organic chemistry, can solve the problems of difficult operation and low yield, and achieve the effects of low cost, increased yield, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Step A:
[0060] In a 2L three-necked flask, add 350ml of water and 350ml of 1,4-dioxane, stir evenly, add 67.3g of L-threonine, 94.5ml of triethylamine, and 181g of di-tert-butyl dicarbonate, react overnight at room temperature, point After the plate reaction is complete, add 500ml of ethyl acetate, stir for 30 minutes, remove the organic layer, adjust the pH of the aqueous layer to 2-3 with hydrochloric acid, extract with ethyl acetate 300×3, combine the organic layers, and dry with anhydrous sodium sulfate. Filtration and concentration gave 115 g of a colorless oily substance with a yield of 93%.
[0061] Step B
[0062] Take 11.2g of the product of step A and dissolve it in a 250ml three-neck flask with 100ml of tetrahydrofuran, cool to -5°C with an ice-salt bath, add 7.2g of 1-hydroxybenzotriazole, and add 11.7g of N,N-dicyclohexylcarbodiimide , stirred for 30 minutes, added 5.5g sulfamic acid and 5.7g triethylamine, reacted at low temperature for 1 hour, removed...
Embodiment 2
[0070] Step A:
[0071] In a 2L three-necked flask, add 350ml of water and 350ml of 1,4-dioxane, stir well, add 67.3g of L-threonine, 94.5ml of triethylamine, dropwise add 144g of benzyl chloroformate, after the dropwise addition, React overnight at room temperature, after the plate reaction is complete, add 500ml of ethyl acetate, stir for 30 minutes, remove the organic layer, adjust the pH of the aqueous layer to 2-3 with hydrochloric acid, extract with ethyl acetate 300×3, combine the organic layers, and use Dry over sodium sulfate, filter, and concentrate to obtain 136 g of a colorless oil, with a yield of 95%.
[0072] Step B
[0073] Take 13g of the product of step A and dissolve it in a 250ml three-necked flask with 100ml of tetrahydrofuran, cool it to -5°C with an ice-salt bath, add 7.2gl-hydroxybenzotriazole, add 11.7g of N,N-dicyclohexylcarbodiimide, Stir for 30 minutes, add 5.5g sulfamic acid and 5.7g triethylamine, react at low temperature for 1 hour, remove the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com