Preparation method of eptifibatide key raw material L-higher arginine
A technology of eptifibatide and arginine, which is applied in the field of preparation of unnatural amino acids, can solve the problems of high cost, low yield, and complicated purification, and achieve the effect of low route cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
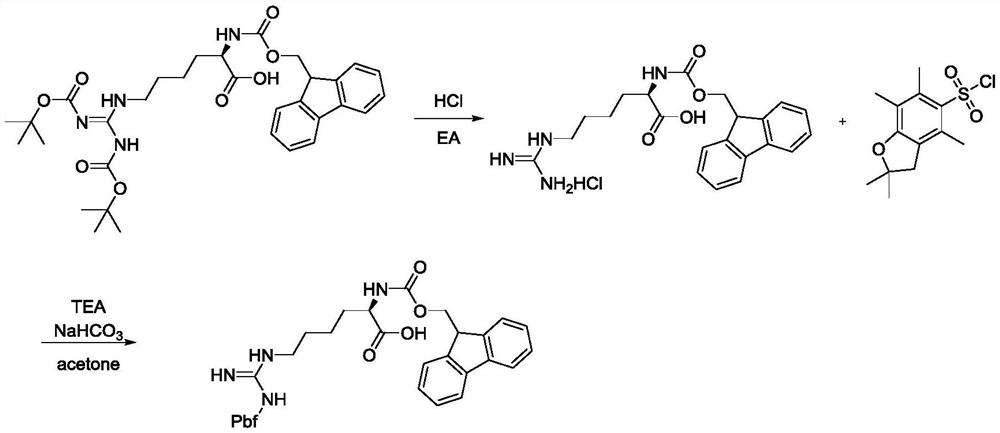
[0030] (S)-11-(tert-butoxycarbonylamino)-1-(9H-fluoren-9-yl)-15,15-dimethyl-3,13-dioxa-2,14-dioxa- Synthesis of 4,10,12-Triazahexadecyl-11-ene-5-carboxylic Acid
[0031]
[0032] 2L tetrahydrofuran was added to a 5L reactor, and 680g (2.622mol, 1eq) of 1,3-di(tert-butoxycarbonyl)guanidine and 291g (2.884mol, 1.2eq) of triethylamine were added under stirring. After the addition was complete, Stir to dissolve, then add 500g (2.623mol, 1eq) of tetrahydrofuran solution of p-toluenesulfonyl chloride dropwise. After extracting with methane and concentrating dichloromethane, a large amount of solids precipitated out, filtered, the filter cake was washed with 500 mL of water, and dried to obtain 950 g of the product. The yield is 87.6%, and the liquid phase content is 99.61%.
[0033]
[0034] In the reaction kettle, add 75Kg DMF, add 7.5Kg (16mol, 1eq) N-fluorenylmethoxycarbonyl-N'-tert-butoxycarbonyl-L-lysine under stirring, after the addition is complete, stir to dissolve, ...
Embodiment 2
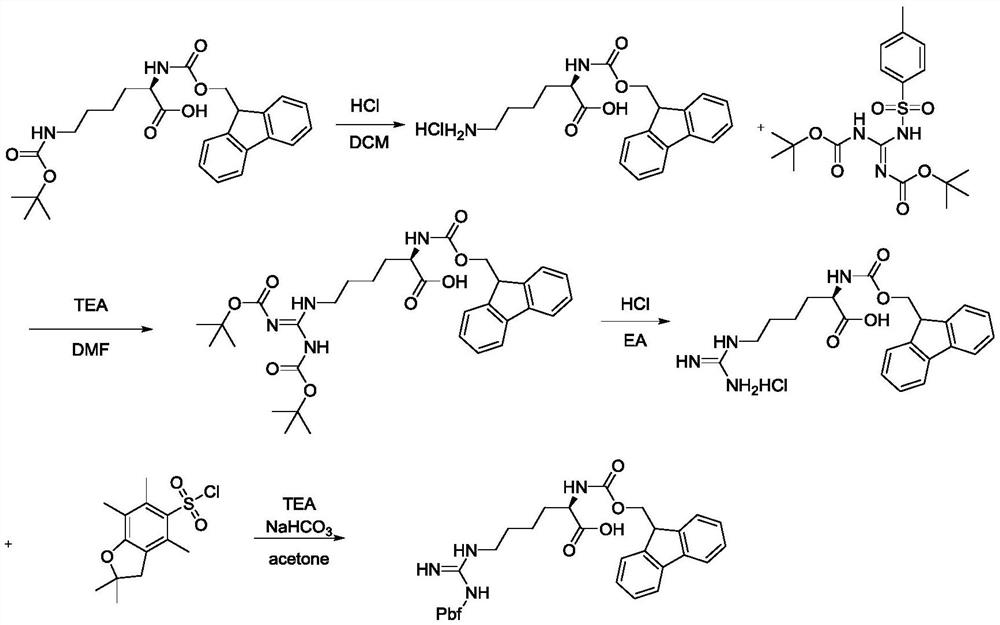
[0036] (S)-11-(tert-butoxycarbonylamino)-1-(9H-fluoren-9-yl)-15,15-dimethyl-3,13-dioxa-2,14-dioxa- Synthesis of 4,10,12-Triazahexadecyl-11-ene-5-carboxylic Acid
[0037]
[0038] Add 3.4L dichloromethane in 5L reactor, add 680g (2.622mol, 1eq) 1,3-bis (tert-butoxycarbonyl) guanidine and 372.8g (2.884mol, 1.2eq) diisopropyl under stirring After the addition of ethylamine, stir to dissolve, then dropwise add 0.5L dichloromethane solution containing 500g (2.623mol, 1eq) p-toluenesulfonyl chloride, control the temperature at 15-20°C, stir and react for 2 hours, take a sample for TLC It was detected that there was no raw material remaining, the solvent was concentrated under reduced pressure and then extracted with dichloromethane. After concentrating the dichloromethane, a large amount of solids precipitated, filtered, the filter cake was washed with 500mL of water, and 1020.2g of the product was obtained after drying. The yield is 94.1%, and the liquid phase UPLC content is 9...
Embodiment 3
[0042] Synthesis of N-(9-fluorenylmethoxycarbonyl)-2,2,4,6,7-pentamethyl-2H-benzofuran-5-sulfonyl-L-arginine
[0043]
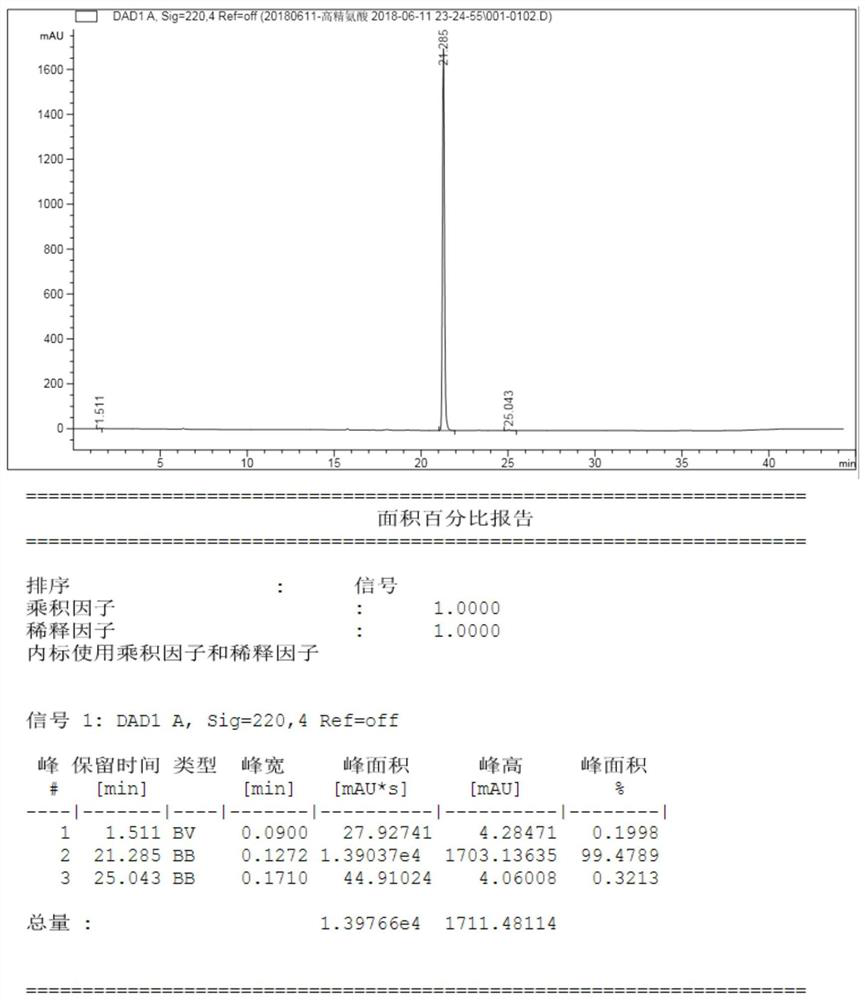
[0044] In the reaction kettle, add 3Kg ethyl acetate, add 500g (0.819mol, 1eq) Fmoc-hArg(Boc2)-OH under stirring, after the addition is complete, stir to dissolve, then cool down to 0°C, add dropwise 4mol / L ethyl acetate Ester hydrochloric acid solution 620mL, control the temperature at 20-25°C, stir and react for 2 hours, concentrate ethyl acetate under vacuum until no liquid, add 1Kg acetone to solidify to obtain 395g wet product of Fmoc-L-arginine hydrochloride (LOD10 %), put 395g wet product Fmoc-L-arginine hydrochloride into the kettle, add 3L acetone, cool to 0°C, add dropwise 8% sodium bicarbonate aqueous solution to adjust pH=7-7.5, add 147.6g (1.142 mol, 1.5eq) DIPEA, add 241.4g (0.836mol, 1.1eq) Pbf-Cl in batches, after the addition is complete, raise the temperature to 20-25°C and react for 3 hours, the raw material is detected by HPLC < 0.2%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com