Synthesis method of trimethoxystilbene
A technology of resveratrol trimethyl ether and a synthesis method, which is applied in the directions of ether preparation, sulfonic acid preparation, sulfonic acid amide preparation, etc., can solve the problems of difficult operation, difficult to scale up production, high price of trifluoromethanesulfonic anhydride, etc. Ease of operation and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
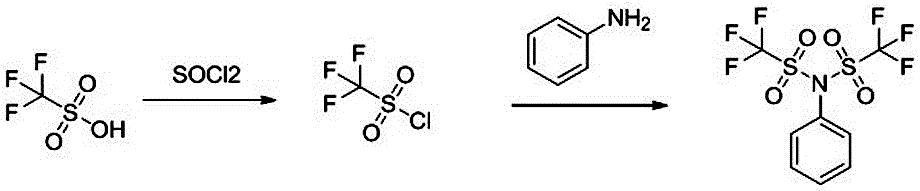
[0016] A synthetic method of resveratrol trimethyl ether, the reaction process is as follows figure 1 As shown, including the following steps:
[0017] 1) At room temperature, add 1 mol of trifluoromethanesulfonic acid to 1L of dichloromethane, stir and slowly add 0.5 mol of thionyl chloride dropwise, control the time for 55 minutes to complete the dropwise addition, stir and react at room temperature for 12 hours, the reaction system Concentrate to obtain crude trifluoromethanesulfonyl chloride, without purification, it can be directly used in the next reaction;
[0018] 2) Add 1 mol of aniline and 0.5 mol of triethylamine to 1L of dichloromethane, cool to 0°C, add 0.5 mol of the crude trifluoromethanesulfonyl chloride obtained in step 1) through a dropping funnel, and add dropwise After the completion, the temperature was raised to room temperature to react for 2 hours, and the reaction system was washed with water, dried, concentrated, and recrystallized with petroleum ether to ...
Embodiment 2
[0020] A synthetic method of resveratrol trimethyl ether, the reaction process is as follows figure 1 As shown, including the following steps:
[0021] 1) At room temperature, add 1 mol of trifluoromethanesulfonic acid to 1L of dichloromethane, stir and slowly add 1.5 mol of thionyl chloride dropwise, control the time for 65 minutes to complete the dropwise addition, stir and react at room temperature for 12 hours, the reaction system Concentrate to obtain crude trifluoromethanesulfonyl chloride, without purification, it can be directly used in the next reaction;
[0022] 2) Add 1 mol of aniline and 1.5 mol of triethylamine to 1L of dichloromethane, cool to 0°C, add 1.5 mol of the crude trifluoromethanesulfonyl chloride obtained in step 1) through a dropping funnel, and add dropwise After the completion, the temperature was raised to room temperature to react for 2 hours, and the reaction system was washed with water, dried, concentrated, and recrystallized with petroleum ether to ...
Embodiment 3
[0024] A synthetic method of resveratrol trimethyl ether, the reaction process is as follows figure 1 As shown, including the following steps:
[0025] 1) At room temperature, add 1 mol of trifluoromethanesulfonic acid to 1L of dichloromethane, stir and slowly add 1 mol of thionyl chloride dropwise, control the time for 60 minutes to complete the dropwise addition, stir at room temperature for 12 hours, and concentrate the reaction system The crude trifluoromethanesulfonyl chloride is obtained without purification and can be directly used in the next reaction;
[0026] 2) Add 1 mol of aniline and 1 mol of triethylamine to 1L of dichloromethane, cool to 0°C, and add 1 mol of the crude trifluoromethanesulfonyl chloride obtained in step 1) through a dropping funnel. After the addition is complete The reaction system was heated to room temperature for 2 hours, and the reaction system was washed with water, dried, concentrated, and recrystallized with petroleum ether to obtain resveratr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com