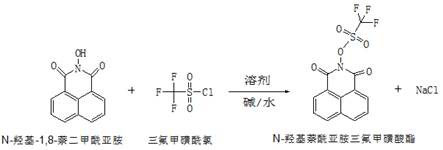
Synthesis method of N-hydroxynaphthalimide trifluoromethanesulfonate
A technology of hydroxynaphthoimide trifluoromethanesulfonate and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of difficult degradation of organic bases, unfavorable recovery, high boiling point, etc., and achieve easy recovery, high product yield, and improved The effect of reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
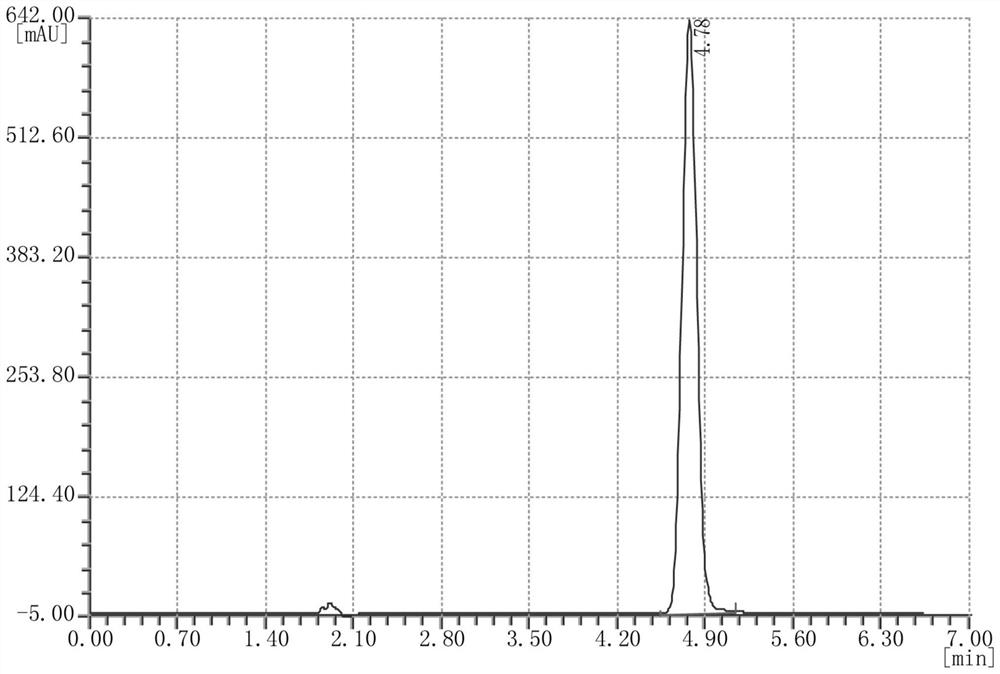
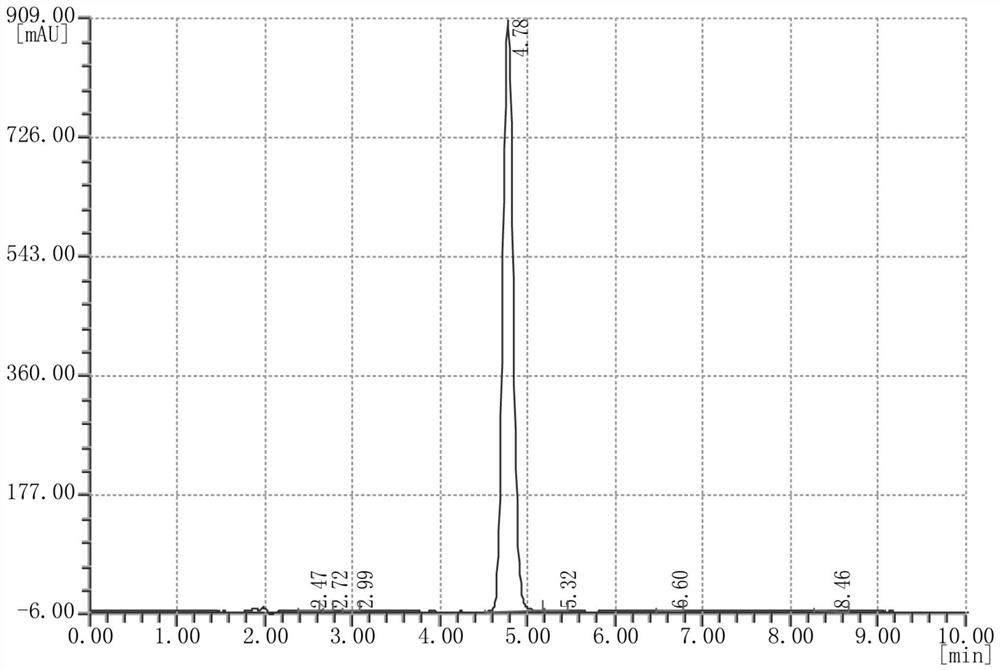
Image
Examples
Embodiment 1
[0027] Add 330 mL of solvent toluene and 100 g of N-hydroxy-1,8-naphthalimide to a 1L four-necked flask equipped with a stirring paddle, a condenser, and a thermometer, start stirring, and cool down to a reaction system temperature of 8°C. Slowly add 79g of trifluoromethanesulfonyl chloride dropwise to the reaction system, and keep the reaction for 6h. Monitor the reaction process. After the reaction is detected by TCL, 134g of NaOH solution with a mass concentration of 14% is added dropwise to the system after the sampling and detection is completed, and it is uniform after stirring for 30min. Under the action of the solvent toluene, the phases are extracted and separated, and after standing and layering, the extracted toluene phase is concentrated under reduced pressure at 55-65°C. The pressure is 0.099MPa-0.1MPa, the temperature is cooled to room temperature, and the wet product obtained by suction filtration is the crude product of N-hydroxynaphthoimide trifluoromethanesul...
Embodiment 2
[0029] Add 330 mL of solvent xylene and 100 g of N-hydroxy-1,8-naphthalimide into a 1L four-necked flask equipped with a stirring paddle, condenser, and thermometer, start stirring, and cool down to a reaction system temperature of 5°C. Slowly add 79g of trifluoromethanesulfonyl chloride dropwise to the reaction system, and keep the reaction for 7h. Monitor the reaction process, and after the sampling and detection is completed, add dropwise Na to the system with a mass concentration of 14%. 2 CO 3 The solution was 177g, and it was homogeneous after stirring for 30 minutes. Under the action of the solvent xylene, the phases are extracted and separated, and after standing and layering, the xylene phase is concentrated under reduced pressure at 65-75°C. MPa-0.1MPa, cool down to room temperature, and get the wet product by suction filtration, which is the crude product of N-hydroxynaphthoimide triflate. The crude product is passed through 4 times the weight of 20% toluene / 80% w...
Embodiment 3
[0031] Add 330mL of solvent benzene and 100g of N-hydroxy-1,8-naphthalimide to a 1L four-necked flask equipped with a stirring paddle, a condenser, and a thermometer, start stirring, and cool down to a reaction system temperature of 10°C. Slowly add 79 g of trifluoromethanesulfonyl chloride dropwise to the reaction system, and keep the reaction for 5 hours. Monitor the reaction process, after sampling and testing, add dropwise 188g of NaOH solution with a mass concentration of 10% to the system, stir for 30min and then make it uniform. Under the action of the solvent benzene, the phases are extracted and separated, and after standing and layering, the benzene phase is concentrated under reduced pressure at 50-60°C. The condition of the reduced-pressure concentration in this example is 55°C, and the pressure of the reduced-pressure concentration is 0.099MPa -0.1MPa, cool down to room temperature, and obtain wet product N-hydroxynaphthoimide trifluoromethanesulfonate crude produ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com