Synthesis method of bisamide insecticide
A synthesis method and bisamide technology are applied in the directions of biocides, biocides, botanical equipment and methods, etc., which can solve the problems of cumbersome reaction steps, low product yield, and many side reactions, and achieve high purity and yield. Good and pollution-reducing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
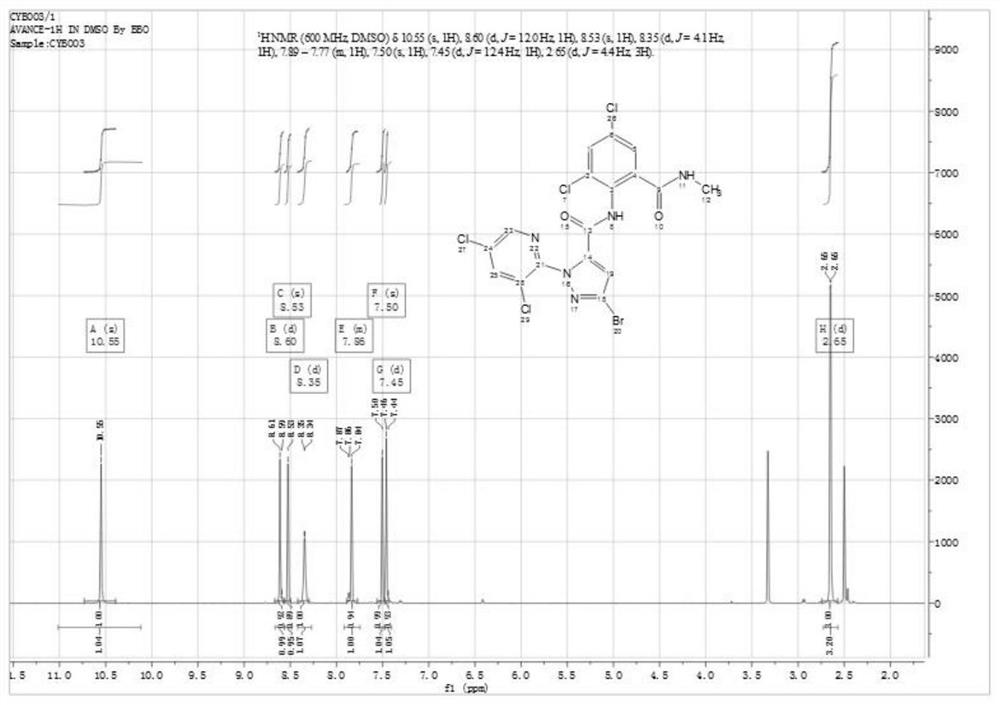
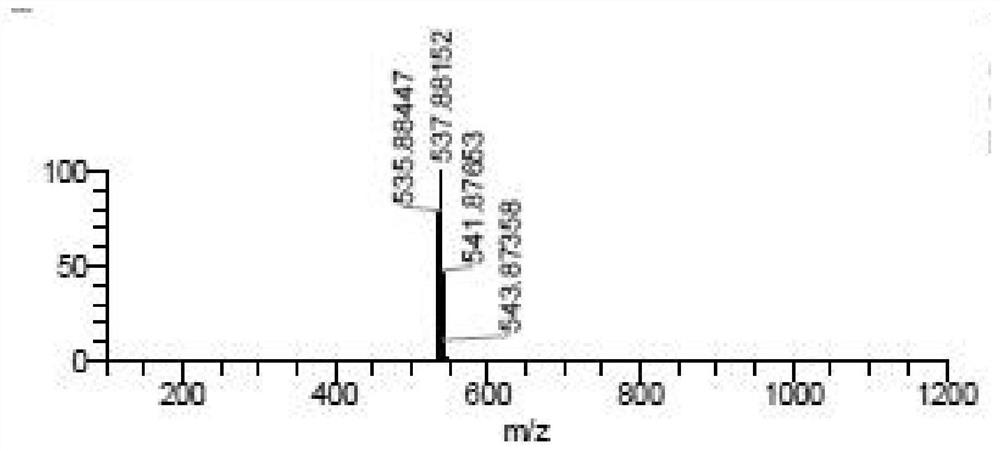
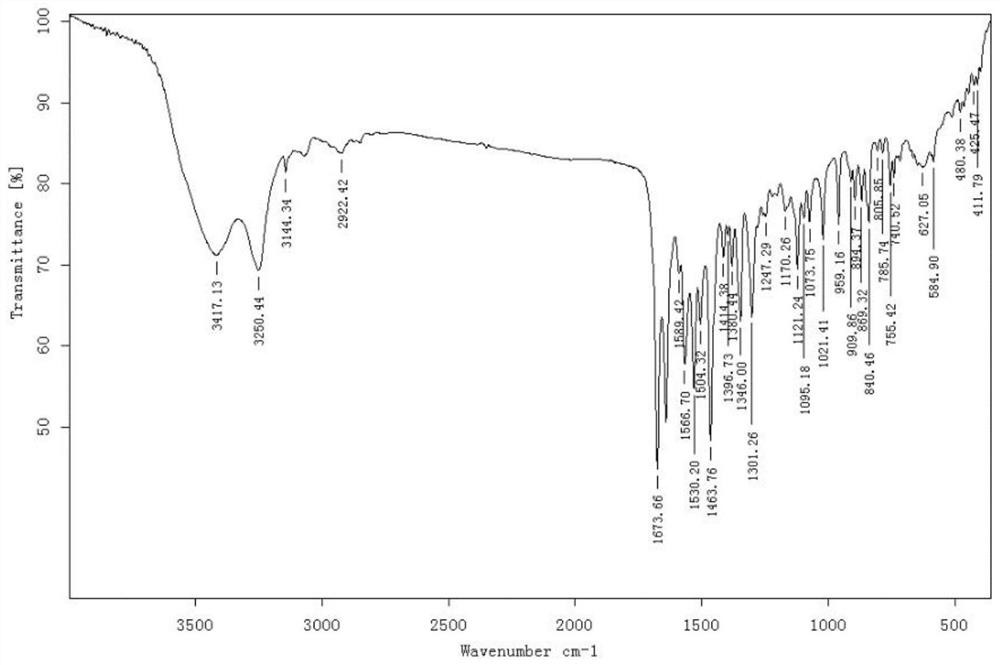
[0039] A kind of synthetic method of bisamide insecticide, described reaction formula is specifically as follows:
[0040]
[0041] The specific steps of the synthetic method are as follows:
[0042] To a 100mL reaction flask, add 3-bromo-1-(3,5-dichloro-2-pyridyl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid (3.57g, 95%, 10 mmol) and acetonitrile (15 mL), the mixture was cooled to -5 °C, and then methanesulfonyl chloride (2.78 g, 99%, 24 mmol) was added dropwise to the reaction flask at -5 °C to 0 °C. After the dropwise addition was completed, stir at room temperature for 10 min, then slowly heat to 50° C., and keep warm for 10 h to obtain a reaction mixture.
[0043] The resulting reaction mixture was cooled to 25°C, 2-amino-3,5-dichlorobenzamide (2.40g, 97%, 10.5mmol) and pyridine (2.24g, 99%, 28mmol) were added, and at this temperature After stirring at low temperature for 3 h, water (5 mL) was added dropwise, and the mixture was stirred at room temperature for 1 h to ob...
Embodiment 2
[0053] To a 100mL reaction flask, add 3-bromo-1-(3,5-dichloro-2-pyridyl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid (3.57g, 95%, 10 mmol) and toluene (15 mL), the mixture was cooled to -5 °C, and then methanesulfonyl chloride (2.78 g, 99%, 24 mmol) was added dropwise to the reaction flask at -5 °C to 0 °C. After the dropwise addition was completed, stir at room temperature for 10 min, then slowly heat to 50° C., and keep warm for 10 h to obtain a reaction mixture.
[0054] The resulting reaction mixture was cooled to 25°C, 2-amino-3,5-dichlorobenzamide (2.40g, 97%, 10.5mmol) and pyridine (2.24g, 99%, 28mmol) were added, and at this temperature After stirring at low temperature for 3 h, water (5 mL) was added dropwise, and the mixture was stirred at room temperature for 1 h to obtain the crude product.
[0055] The crude product was filtered, and the solid was washed with 3:1 toluene-water (2×3 mL) and then with toluene (3 mL), and the solid was filtered and dried to obtain the...
Embodiment 3
[0059] To a 100mL reaction flask, add 3-bromo-1-(3,5-dichloro-2-pyridyl)-4,5-dihydro-1H-pyrazole-5-carboxylic acid (3.57g, 95%, 10 mmol) and acetonitrile (15 mL), the mixture was cooled to -5 °C, and then methanesulfonyl chloride (2.78 g, 99%, 24 mmol) was added dropwise to the reaction flask at -5 °C to 0 °C. After the dropwise addition was completed, stir at room temperature for 10 min, then slowly heat to 50° C., and keep warm for 10 h to obtain a reaction mixture.
[0060] The resulting reaction mixture was cooled to 25°C, and 2-amino-3,5-dichlorobenzamide (2.40g, 97%, 10.5mmol) and triethylamine (2.86g, 99%, 28mmol) were added. After stirring at this temperature for 3 h, water (5 mL) was added dropwise, and the mixture was stirred at room temperature for 1 h to obtain a crude product.
[0061] The crude product was filtered, and the solid was washed with 3:1 acetonitrile-water (2×3 mL) and then with acetonitrile (3 mL), the solid was filtered and dried to obtain a bisami...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com