2-aryl-3-methyl benzofuran-benzimidazole salt compound and preparation method thereof
A kind of salt compound, technology of benzimidazole, applied in the application field of anti-tumor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
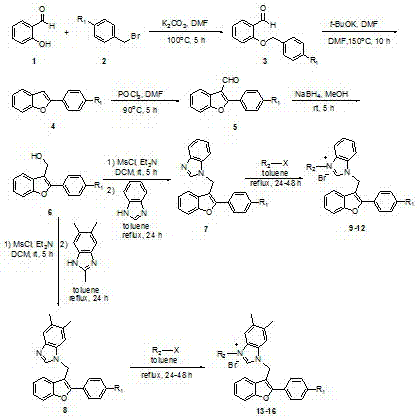
[0019] The preparation method specifically includes:
[0020] A, the preparation of compound benzyl ether salicylaldehyde:
[0021] Using salicylaldehyde as raw material, synthesize benzyl ether salicylaldehyde with 4-substituted benzyl bromide in anhydrous DMF: dissolve salicylaldehyde, 4-bromobenzyl bromide or 4-trifluoromethyl benzyl bromide in anhydrous DMF , adding potassium carbonate, the dosage is salicylaldehyde in molar ratio: 4-substituted benzyl bromide: potassium carbonate = 1: 1: 2, the dosage of anhydrous DMF is 30 ml: 1g salicylaldehyde, stirred at 100°C for 10 After 1 hour, cool to room temperature, filter with suction, add ethyl acetate to dilute (50 ml: 1g substrate), wash with water (50 ml) and saturated brine (50 ml) respectively, and wash the organic phase with anhydrous MgSO 4 After drying, filtering, and concentrating the solvent under reduced pressure, perform silica gel column chromatography, using petroleum ether: ethyl acetate (30: 1) as the eluent,...
Embodiment 1
[0033] Preparation of compound 9: see the above preparation methods A, B, C, D, E, F.
[0034]
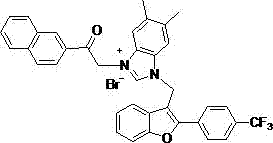
[0035] Compound 9: Molecular Formula C 31 h 24 Br 2 N 2 o 3 , yield 63%, white solid. IR ν max (cm -1): 3436,3329, 3137, 2921, 1680, 1606, 1446, 1248, 1178, 1120, 1025, 984, 833, 754,699 cm -1 . 1 H-NMR (300 MHz ,DMSO) δ: 9.79 (1H, s), 8.09-8.04 (3H, m), 7.94-7.70 (6H, m), 7.69-7.43 (3H, m), 7.42-7.39 (1H , t, J=4.5 Hz), 7.33-7.28 (1H, t , J=7.5 Hz), 7.17-7.14 (2H, d, J=8.7 Hz), 6.31 (2H, s), 6.24 (2H, s) , 3.88 (3H,s). 13 C-NMR (75 Hz, DMSO) Δ: 189.30, 164.13, 154.37, 153.54, 143.06, 132.28,132.10, 130.80, 130.28, 129.40, 129.64, 127.46.98, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126.72, 126. , 123.68, 123.45, 119.78, 114.26, 114.10, 113.69, 111.53, 108.10, 107.42, 55.77, 52.93, 41.73. 31 h 24 BrN 2 o 3 + [M-Br] + 551.0965, found: 551.0923.
Embodiment 2
[0037] Preparation of compound 10: see the above preparation methods A, B, C, D, E, F.
[0038]
[0039] Compound 10: Molecular Formula C 34 h 24 Br 2 N 2 o 2 , yield 80%, white solid. IR ν max (cm -1 ): Yield 80%, white solid, IR(KBr): 3428, 3321, 3126, 2917, 1686, 1615, 1564, 1446, 1264,1188, 1124, 1071, 1006, 822, 754, 696 cm -1 . 1 H-NMR (300 MHz, DMSO) δ: 9.84 (1H, s), 8.92 (1H, s), 8.22-8.01 (5H, m), 7.97-7.81 (5H, m), 7.75-7.65 (6H, m ),7.64-7.59 (1H, t, J=7.5 Hz), 7.43-7.32 (1H, m), 6.54 (2H, s), 6.28 (2H, s). 13 C-NMR (75 Hz, DMSO) Δ: 191.04, 154.39, 153.55, 143.09, 135.50, 132.30,131.97, 130.88, 130.01, 129.99, 129.33, 128.05,127.8.8.7.48, 127.7.7.7.7.7.7.7.7.7.8 , 125.68, 123.71, 123.27, 119.81,114.17, 113.73, 111.55, 107.43, 53.41, 41.79. HRMS (ESI-TOF) m / z: Calcd for C 34 h 24 BrN 2 o 2 + [M-Br] + 571.1016, found: 571.1054.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com