Preparation method of (S)-2-methyl azetidine hydrochloride
A technology of methylazetidine and hydrochloride, applied in the direction of organic chemistry methods, chemical instruments and methods, organic racemization, etc., can solve the problems of unfavorable industrial production, high cost of raw materials, complicated operation, etc., and achieve simplification The effect of operation, low raw material price and high reaction yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
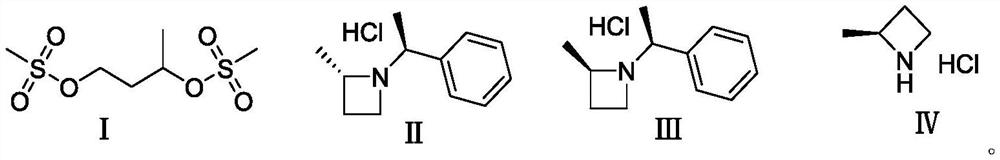
[0047] The preparation method comprises the following steps:
[0048] Step 1), the raw material 1,3-butanediol reacts with methanesulfonyl chloride in a reaction solvent in the presence of a base to obtain a compound of formula (I);
[0049] Step 2), the ring-closing reaction of the compound of formula (I) and S-phenethylamine to obtain a mixture of the compound of formula (II) and the compound of formula (III);
[0050] Step 3), the mixture of the compound of formula (II) and the compound of formula (III) is refined and resolved to obtain the compound of formula (II);
[0051] Step 4), the compound of formula (II) is dissolved in a solvent, and deprotected under the action of a catalyst to obtain the compound of formula (IV).
[0052] The synthetic route is as follows:
[0053]
Embodiment 1
[0055]
[0056] Add 1,3-butanediol (90 g, 1 mol), triethylamine (130 g, 1.3 mol) and DCM (1 L) into the reaction flask, and add methanesulfonyl chloride (350 g, 3 mol) dropwise at room temperature. After the dropwise addition was completed, the reaction was carried out at room temperature for 2h. After the reaction was completed, saturated sodium chloride solution was added to wash with water, extracted with DCM (3*500mL), the organic phase was dried over anhydrous sodium sulfate, concentrated to obtain a crude product, and the crude product was passed through a column to obtain 220 g of the compound of formula (I) (yield 91%). 1 H NMR (CDCl 3)δ: 1.48-1.50 (d, 3H), 2.06-2.10 (m, 2H), 3.03-3.05 (s, 6H), 4.32-4.35 (t, 2H), 4.96-5.00 (m, 1H).
Embodiment 2
[0058]
[0059] Add S-phenylethylamine (1187g, 9.8mol) into the reaction flask, control the temperature below 45°C, add the compound of formula (I) (690g, 2.8mol) dropwise, stir at room temperature for 1h after the addition, then raise the temperature to 45°C for 10h ( A large amount of solids were precipitated), and the ratio of the diastereomer formula (II) compound and the formula (III) compound detected by LC was 91:9. After the reaction was completed, filter, combine the organic phases, wash and separate the liquids, add HCl / EA (1.5L) The solution was stirred to form a salt, filtered, MTBE recrystallized, filtered and dried to obtain 486 g of the compound of formula (II) (yield 82%).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com