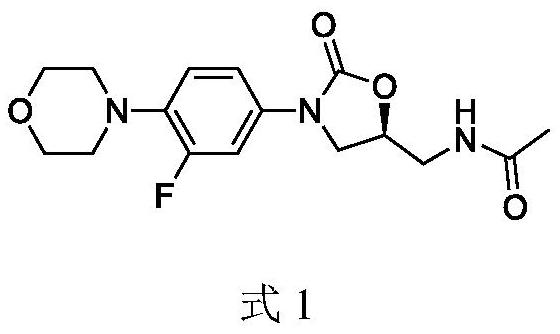
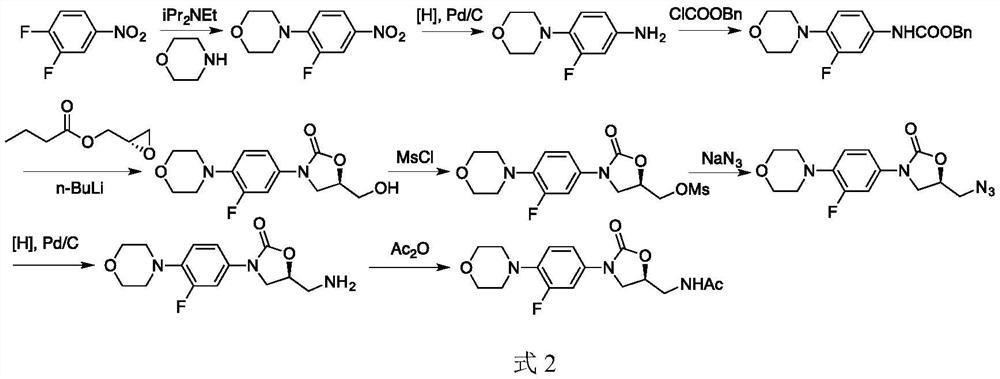
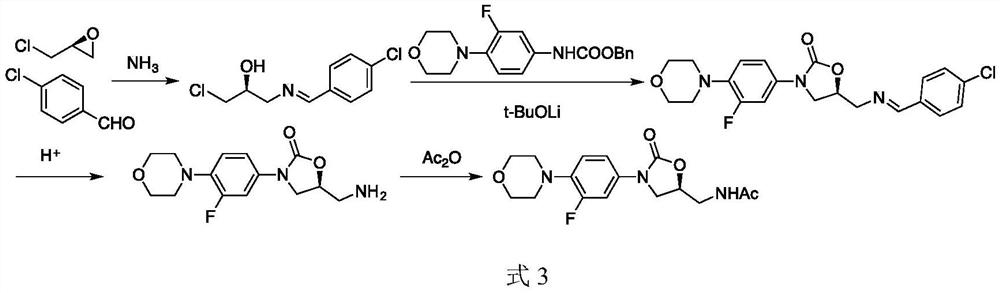
A kind of preparation method of linezolid intermediate
A technology for linezolid and intermediates, applied in the field of preparation of linezolid intermediates, to achieve the effects of reduced production costs, low prices, and high product yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation step of the intermediate N-(3-phthalimido-2-(S)-hydroxypropyl)-3-fluoro-4-(morpholinyl)aniline (I) of linezolid is as follows:
[0035] Preparation of raw materials: N,N-dimethylacetamide is the amide dipolar solvent, and sodium bromide is selected as the reaction accelerator;
[0036] The (II):(III) molar ratio was 2:1.
[0037] (1) In a 250 ml reaction flask, dissolve 4.0 g (II) in 30 ml of N,N-dimethylacetamide solvent, add 2.4 g (III) and 1.0 g sodium bromide, stir for 20 min, and heat up to Under the condition of 100℃, react for 20h to obtain a reaction solution;
[0038] (2) The reaction solution obtained in step (1) was cooled to room temperature; 50ml of dichloromethane and 50ml of water were added, and the liquid was stirred and separated, 50ml of water was added to the organic phase, washed 3 times, and the organic phase was dried with anhydrous magnesium sulfate, The crude product was obtained by concentration, and the crude product was sepa...
Embodiment 2
[0040] The preparation step of the intermediate N-(3-phthalimido-2-(S)-hydroxypropyl)-3-fluoro-4-(morpholinyl)aniline (I) of linezolid is as follows:
[0041]Preparation of raw materials: N,N-dimethylacetamide is the amide dipolar solvent, and sodium bromide is selected as the reaction accelerator;
[0042] The (II):(III) molar ratio was 1.77:1.
[0043] (1) In a 250 ml reaction flask, dissolve 3.5 g of (II) in 30 ml of N,N-dimethylacetamide solvent, add 2.4 g of (III) and 1.5 g of sodium bromide, stir for 20 min, and heat up to Under the condition of 100℃, react for 20h to obtain a reaction solution;
[0044] (2) The reaction solution obtained in step (1) was cooled to room temperature; 50ml of dichloromethane and 50ml of water were added, and the liquid was stirred and separated, 50ml of water was added to the organic phase, washed 3 times, and the organic phase was dried with anhydrous magnesium sulfate, The crude product was obtained by concentration, and the crude produ...
Embodiment 3
[0046] The preparation step of the intermediate N-(3-phthalimido-2-(S)-hydroxypropyl)-3-fluoro-4-(morpholinyl)aniline (I) of linezolid is as follows:
[0047] Preparation of raw materials: N,N-dimethylacetamide is the amide dipolar solvent, and tetrabutylammonium bromide is selected as the reaction accelerator;
[0048] The (II):(III) molar ratio was 1.83:1.
[0049] (1) In a 250ml reaction flask, dissolve 3.6g (II) in 30ml of N,N-dimethylacetamide solvent, add 2.4g (III) and 1.5g tetrabutylammonium bromide, stir for 25min , the temperature was raised to 120 °C, and the reaction was carried out for 21 h to obtain a reaction solution;
[0050] (2) The reaction solution obtained in step (1) was cooled to room temperature; 50ml of dichloromethane and 50ml of water were added, and the liquid was stirred and separated, 50ml of water was added to the organic phase, washed 3 times, and the organic phase was dried with anhydrous magnesium sulfate, The crude product was obtained by c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com