Preparation method of dronedarone hydrochloride and intermediate of dronedarone hydrochloride
A technology for dronedarone hydrochloride and intermediates, which is applied in the field of pharmaceutical preparation, can solve the problems of complex operation, cumbersome reaction, and long process time, and achieve the effects of simple preparation process, simple purification process, and easy removal process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
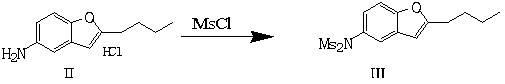
[0062] Take 225.7g (1mol) of 2-butyl-5-aminobenzofuran hydrochloride, add it into 2257ml of dichloromethane, stir and cool down to below 10°C, add 404g (4mol) of triethylamine within 20min, and then Add 331.8g (3 mol) of methanesulfonyl chloride dropwise at a temperature of 10-20°C, and the dropwise addition is completed in about 30 minutes. After the dropping is completed, stir for 30 minutes, filter with suction, wash the filter cake with 200ml of dichloromethane, combine the filtrates, and evaporate to dryness to obtain a solid. The solid was beaten with 1000ml of 5wt% hydrochloric acid for 30min, suction filtered, the filter cake was washed with 50ml of water, and dried to obtain 350g of crude product. Add the crude product to 1750ml of absolute ethanol, heat to reflux for 30min, then cool down to 0°C and stir for 30min, then suction filter, wash the filter cake with 20ml of absolute ethanol, dry the filter cake to obtain 2-butyl-5-[( N,N-Dimethylsulfonyl)amino]benzofuran ...
Embodiment 2
[0064] Take 1mol of 2-butyl-5-aminobenzofuran hydrochloride, add it to 2500ml of chloroform, stir and cool down to below 10°C, add 5mol of sodium carbonate within 20min, and then add formaldehyde dropwise at a temperature of 10-20°C Add 4 mol of sulfonyl chloride in about 30 minutes. After the dropping, stir for 30 minutes, filter with suction, wash the filter cake with 250 ml of chloroform, combine the filtrates, and evaporate to dryness to obtain a solid. Slurry the solid with 1000ml of 5wt% hydrochloric acid for 30min, filter with suction, wash the filter cake with 50ml of water, and dry to obtain the crude product. Add the crude product to 1750ml of absolute ethanol, heat to reflux for 30min, then cool down to 0°C and stir for 30min, then suction filter, wash the filter cake with 20ml of absolute ethanol, dry the filter cake to obtain 2-butyl-5-[( N,N-Dimethylsulfonyl)amino]benzofuran, the yield was 87%.
Embodiment 3
[0066] Take 1mol of 2-butyl-5-aminobenzofuran hydrochloride, add it to 2000ml of benzene, stir and cool down to 0-10°C, add 3mol of potassium carbonate within 20min, then add methanesulfonate dropwise at a temperature of 10-20°C Acyl chloride 2mol, about 30 minutes to complete the dropwise addition, stirring for 30 minutes after the completion of the dropping, suction filtration, the filter cake was washed with 200ml of benzene, the combined filtrates were evaporated to dryness to obtain a solid. Slurry the solid with 950ml of 5wt% hydrochloric acid for 30min, filter with suction, wash the filter cake with 50ml of water, and dry to obtain the crude product. Add the crude product to 1700ml of absolute ethanol, heat to reflux for 30min, then cool down to 0°C and stir for 30min, then suction filter, wash the filter cake with 20ml of absolute ethanol, dry the filter cake to obtain 2-butyl-5-[( N,N-Dimethylsulfonyl)amino]benzofuran, the yield was 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com