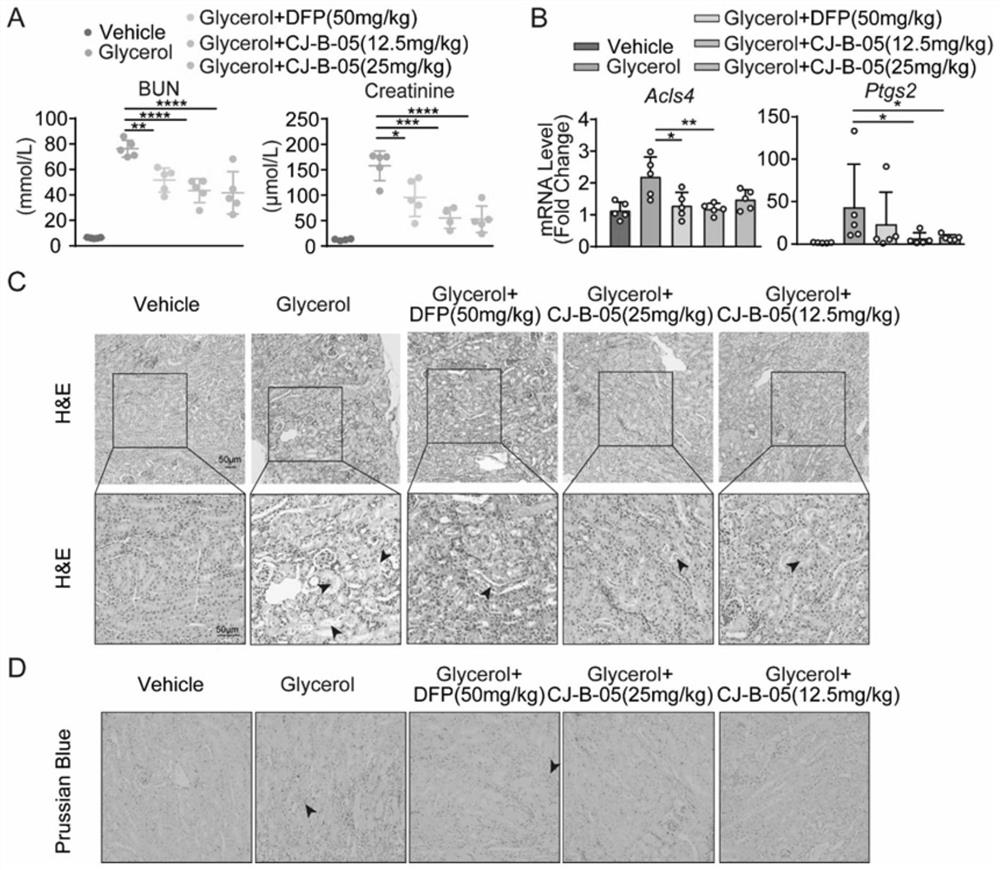
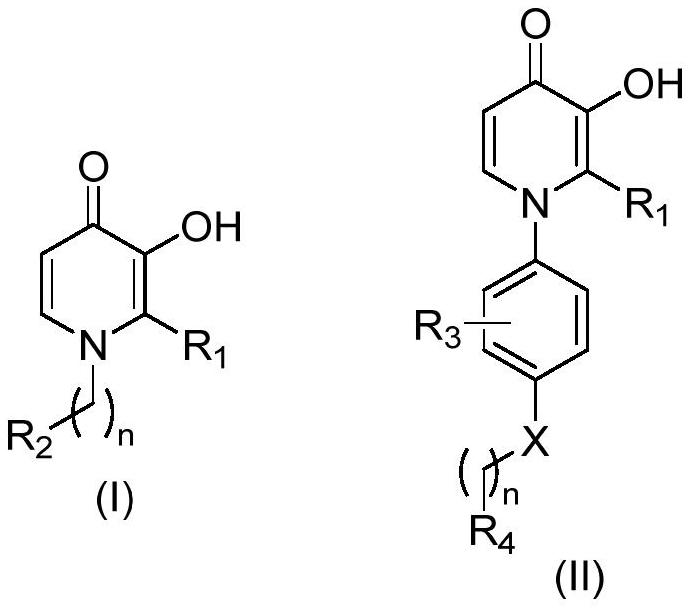
3-hydroxypyridine-4-ketone derivative and application thereof in inhibition of renal cell ferroptosis
A technology of hydroxypyridine and picoline, which is applied in the field of medicinal chemistry to achieve the effects of alleviating acute kidney injury, reducing blood urea nitrogen and serum creatinine levels, and inhibiting renal cell ferroptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Synthesis of 3-hydroxy-2-methyl-1-phenethylpyridine-4(1H)-one hydrochloride (number CJ-B-01)
[0026]
[0027] 126 mg (1 mmol) of 3-hydroxy-2-methyl-4H-pyrone and 122 mg (1 mmol) of phenylboronic acid were added to the reaction flask, 5 mL of water was added, the mixture was heated to 80 degrees, and 132 μL (1.05 mmol) of phenethylamine was added. , continue to stir the reaction at 80 degrees, and TLC detects the reaction (dichloromethane / methanol=20:3 volume ratio). After the reaction, the reaction solution was spin-dried by a rotary evaporator, 5 mL of concentrated hydrochloric acid was added and stirred at room temperature for 6 h to form a salt, concentrated by rotary evaporation, and 3 mL of acetonitrile was added to obtain the precipitate and suction filtration. Salt product 252 mg, yield 95%.
[0028] White solid, mp: 245.9-246.8℃; 1 H NMR (500MHz, DMSO-d 6 )δ10.40(s, 1H), 8.12(d, J=7.0Hz, 1H), 7.34-7.28(m, 3H), 7.25-7.20(m, 3H), 4.58(t, J=7.4Hz,...
Embodiment 2
[0029] Example 2. Synthesis of 2-ethyl-3-hydroxy-1-phenethylpyridine-4(1H)-one hydrochloride (number CJ-B-02)
[0030]
[0031] 3-hydroxy-2-ethyl-4H-pyrone was used instead of 3-hydroxy-2-methyl-4H-pyrone, the molar weight was unchanged, and the rest were the same as in Example 1, to obtain 257 mg of product, yield 92% .
[0032] White solid, mp: 201.7-202.3℃; 1 H NMR (500MHz, DMSO-d 6 )δ10.53(s,1H),8.12(d,J=7.0Hz,1H),7.31-7.29(m,3H),7.26-7.23(m,1H),7.21-7.20(m,2H),4.56 (t, J=7.4Hz, 2H), 3.11 (t, J=7.3Hz, 2H), 2.86 (q, J=7.4Hz, 2H), 1.14 (t, J=7.5Hz, 3H); 13 C NMR (125MHz, DMSO-d 6 )δ159.0,145.7,142.8,138.2,136.2,129.1,128.7,127.1,110.8,56.2,40.0,39.9,39.7,39.5,39.4,39.2,39.0,36.5,19.6,11.9; HRMS(ESI): m / z calcd forC 15 H 18 NO 2 [M+H] + :244.1332,found:244.1338.
Embodiment 3
[0033] Example 3. Synthesis of 1-(cyclohexylmethyl)-3-hydroxy-2-methylpyridin-4(1H)-one hydrochloride (number CJ-B-03)
[0034]
[0035] The phenethylamine was replaced with cyclohexylmethylamine, the molar weight was unchanged, and the rest were the same as those in Example 1, to obtain 247 mg of the product, with a yield of 96%.
[0036] White solid, mp: 188.7-189.6℃; 1 H NMR (500MHz, DMSO-d 6 )δ10.54(s, 1H), 8.20(dd, J=8.0, 3.0Hz, 1H), 7.40(d, J=6.9Hz, 1H), 4.20(d, J=7.5Hz, 2H), 2.53( s, 3H), 1.80–1.73 (m, 1H), 1.67–1.60 (dd, J=26.1, 9.6Hz, 3H), 1.48–1.46 (d, J=11.7Hz, 2H), 1.20–1.08 (m, 3H), 1.05–0.98 (m, 2H); 13 C NMR (125MHz, DMSO-d 6)δ158.5,143.0,141.5,138.6,110.3,61.2,40.0,39.9,39.7,39.5,39.4,39.2,39.0,37.2,29.3,25.7,25.0,12.8; HRMS(ESI):m / z calcd for C 13 H 20 NO 2 [M+H] + :222.1489,found:222.1485.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com