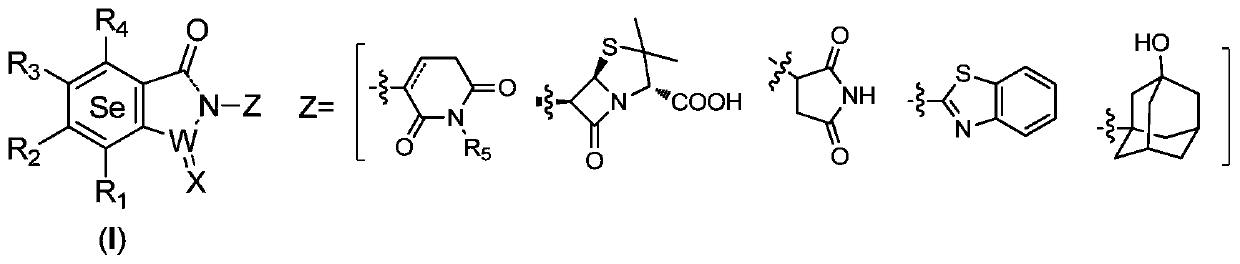
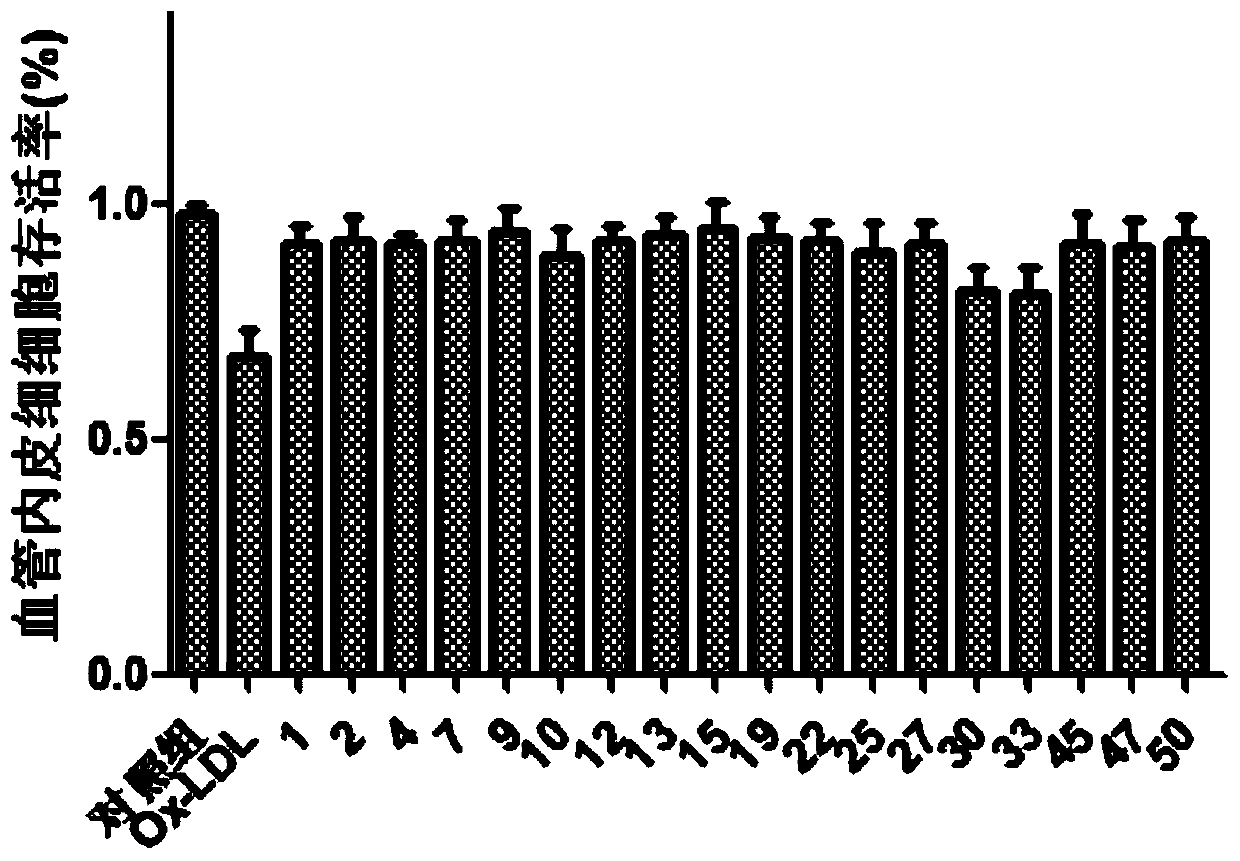
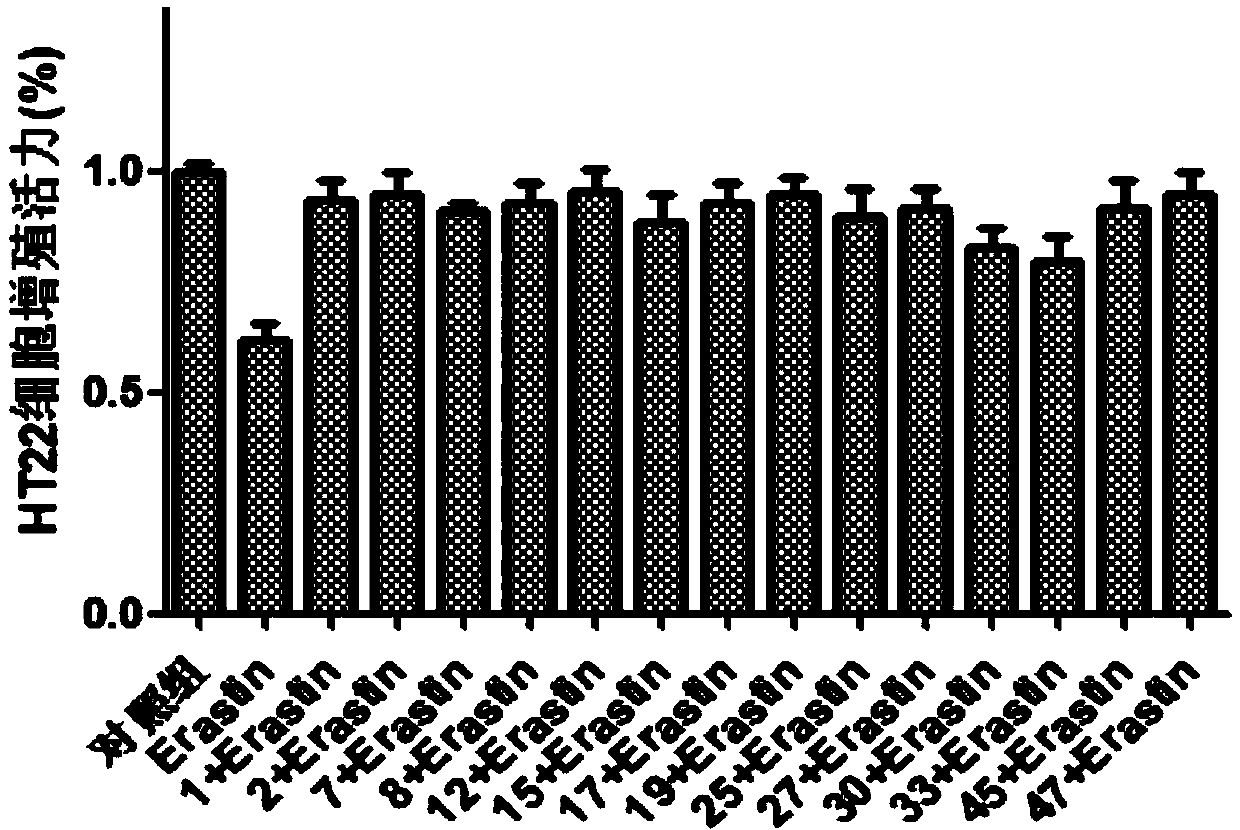
Selenium-containing isoxazolyl amine compound and preparation method and application thereof
A compound, azolamide technology, applied in the field of medicine, can solve problems such as long-term use limitation and reduction of comprehensive therapeutic index
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Synthesis of Example 1 Compound 1
[0085] Preparation Method Step 1: Preparation of 2-Chloroselenyl Benzoyl Chloride
[0086] Anthranilic acid (1.37 g, 10 mmol) was added to 3 N hydrochloric acid aqueous solution (4 ml) under ice-cooling, and then an aqueous solution containing 690 mg of sodium nitrite (10 mmol) was slowly added dropwise with stirring 2 ml, react for 1 hour until clarified, and set aside.
[0087] Under the protection of nitrogen, 790 mg (10 mmol) of selenium powder and 20 mg of cetyltrimethylammonium bromide were weighed and added to 2 N aqueous sodium hydroxide solution (5 ml) to obtain a Se-NaOH solution. Then put NaOH (40 mg, 1 mmol) and NaBH under ice bath 4 (49 mg, 1.3 mmol) in water (1 ml) was added to the above Se-NaOH solution, and stirred at room temperature for 1 hour, then warmed to 90 o C continued to stir and react for half an hour to obtain a sodium diselenide solution. After cooling to room temperature, the 2-benzoic acid diazonium ...
Embodiment 22
[0095] The synthesis of embodiment 22 compound 19
[0096] Synthetic route 1:
[0097]
[0098] Preparation step 1 : intermediate a19 synthesis
[0099] Add o-iodine slowly to a THF solution (10 mL) containing 3-amino-1-adamantanol (167 mg, 1 mmol) and triethylamine (151 mg, 1.5 mmol) under stirring in an ice bath Benzoyl chloride (266 mg, 1 mmol) and continue to react for 1~2 h to complete. After the reaction was detected by TLC, 20 ml of water was added, followed by ethyl acetate (20 mL×2) for extraction, the organic phase was washed successively with saturated brine, dried over anhydrous sodium sulfate, filtered, and the obtained filtrate was evaporated to dryness under reduced pressure and passed through a silica gel column. Chromatographic purification (V 乙酸乙酯 :V 石油醚 =1:4~1:1), the reaction was quantified to obtain a19. MS-ESI [M+H] + 398.0 (397.0).
[0100] Preparation step 2 : Synthesis of Compound 19
[0101] Containing a19 (397 mg, 1 mmol), selenium po...
Embodiment 30
[0104] Example 30 Synthesis of Compound 3
[0105] Add 10% Pd / C to a methanol solution (1 mL) containing compound 2 (72 mg, 0.2 mmol), then place under H 2 Heated to 70°C in the atmosphere for 48 hours to complete the reaction. After the reaction was detected by TLC, it was filtered, and the obtained filtrate was evaporated to dryness under reduced pressure and purified by column chromatography to obtain compound 6 (25 mg, 36%). HRMS-ESI: m / z calcd for C 12 h 10 N 2 o 3 Se:324.9966, found [M+H] + 326.0040; 1 H NMR (400 MHz, DMSO- d 6 ) δ 10.97 (s, 1H), 7.47– 7.34 (m, 1H), 7.05 – 6.83 (m, 2H), 6.33 (brs, 2H), 5.23 (dd, J = 12.8, 5.3Hz, 1H), 2.92 – 2.83 (m, 1H), 2.59 (d, J = 17.3 Hz, 1H), 2.45 – 2.35 (m, 1H), 2.12– 2.02 (m, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com