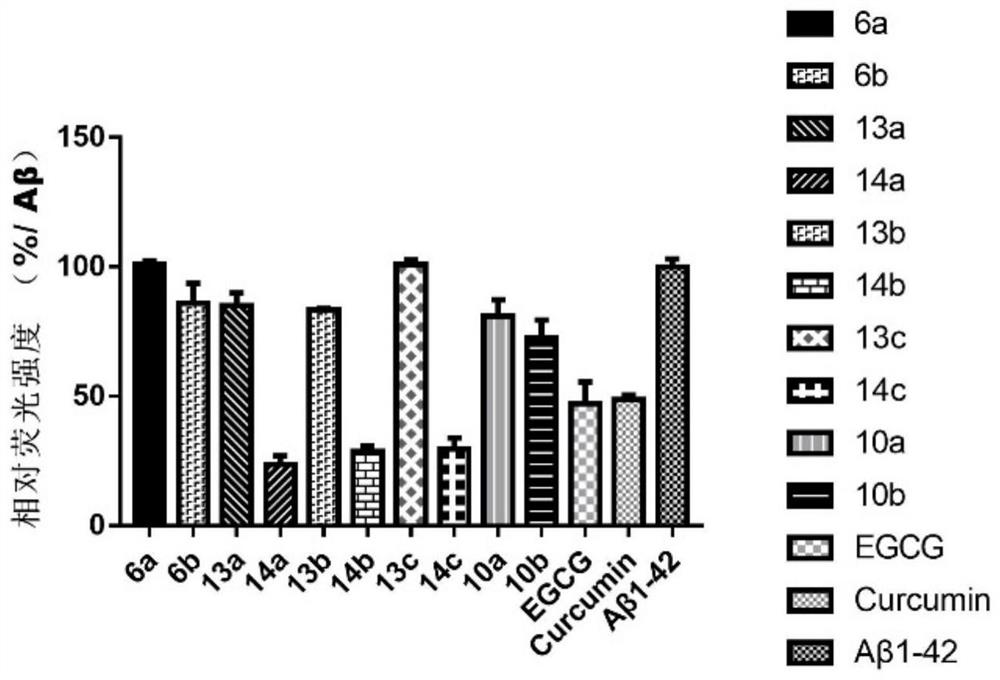
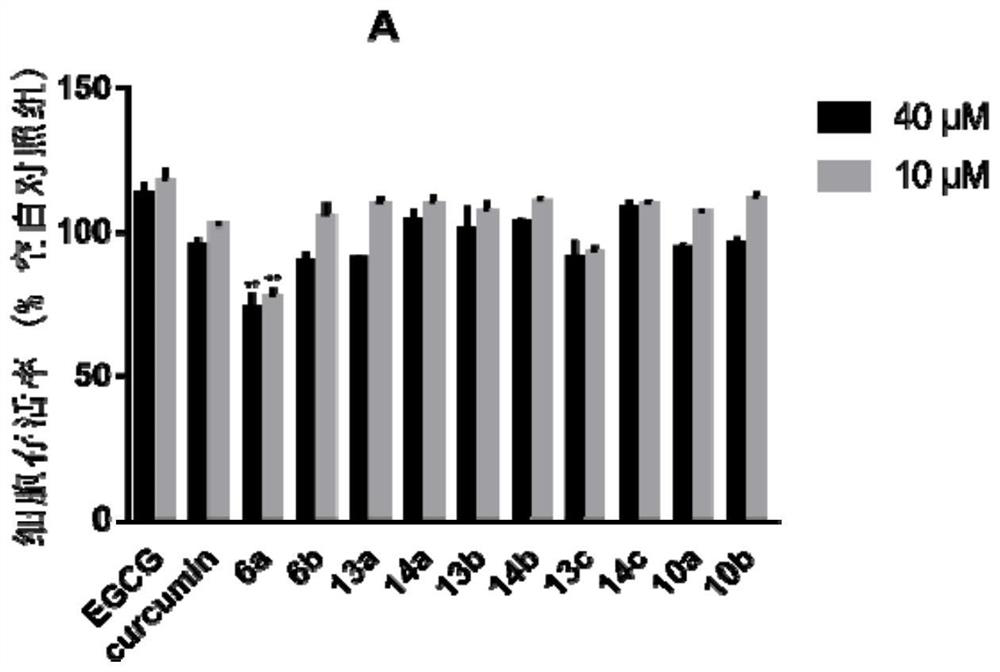
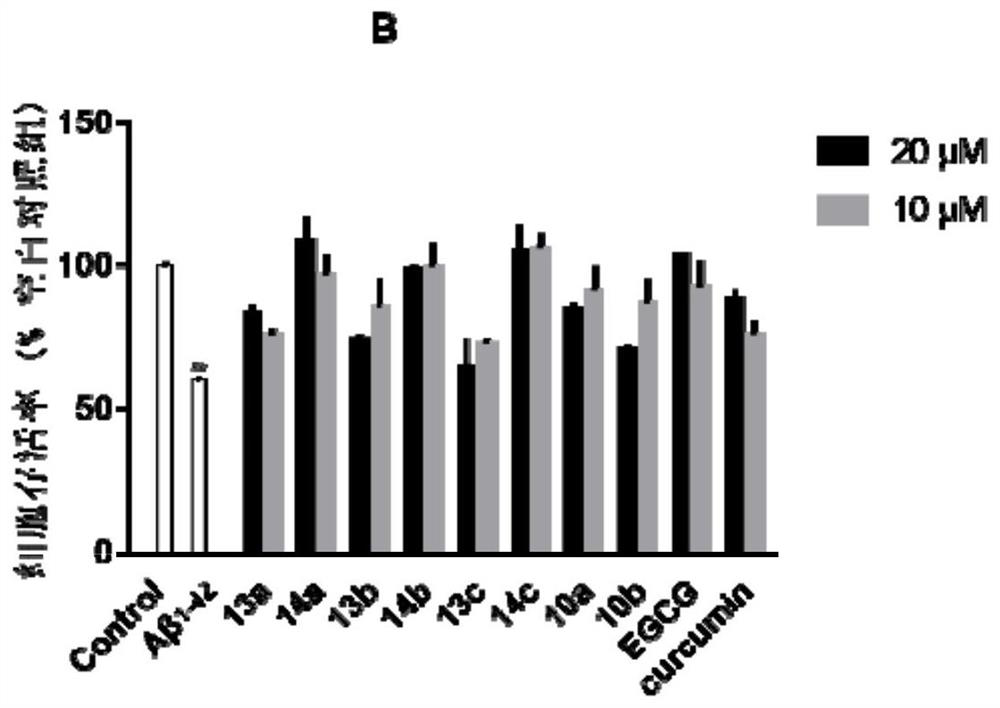
A kind of polyhydroxychalcone compound and its application
A technology of polyhydroxychalcone and methylchalcone, which is applied in the field of medicinal chemistry, can solve problems such as the increase of Aβ protein, achieve a good inhibitory effect, and inhibit the effect of cell ferroptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029]
[0030] Preparation of (E)-3'-hydroxy-3-methylchalcone (6a)
[0031] Add 1.36g (0.01mol) 3-hydroxyacetophenone, 20mL ethanol and 10mL KOH (10%) aqueous solution in a 50mL three-necked flask, stir at room temperature to make it fully dissolve, then add 1.2g (0.01mol) 3 -Methylbenzaldehyde, stirred at room temperature for 24h. After the completion of the reaction was monitored, 10% HCl was added dropwise to acidify to PH = 6, extracted three times with 30 mL ethyl acetate, combined, and then washed with 20 mL saturated brine, the ethyl acetate phase was concentrated by rotary evaporation, and dried to obtain a yellow solid. After column chromatography (200-400 mesh silica gel), 1.77 g of yellow powder solid (6a) was obtained, with a yield of 74.2%.
[0032] After testing, 1 H NMR is: 1 H NMR (700MHz, CDCl 3 )7.82(d, J=15.7Hz, 1H), 7.72(dd, J=2.3, 1.7Hz, 1H), 7.59(d, J=7.7Hz, 1H), 7.52(d, J=15.7Hz, 1H) , 7.44(s,1H),7.43(d,J=7.7Hz,1H),7.39(t,J=7.9Hz,1H),7.31(t,J=7....
Embodiment 2
[0037]
[0038] Preparation of (E)-3'-hydroxy-3,4-dimethoxychalcone (6b)
[0039] Add 1.36g (0.01mol) of 3-hydroxyacetophenone, 20mL of ethanol and 10mL of KOH (10%) in a 50mL three-necked flask, stir at room temperature to fully dissolve, then add 1.66g (0.01mol) of 3 , 4-dimethoxybenzaldehyde, stirred at room temperature for 24h. After the completion of the reaction was monitored, 10% HCl was added dropwise to acidify to PH = 6, extracted three times with 30 mL ethyl acetate, combined, and then washed with 20 mL saturated brine, the ethyl acetate phase was concentrated by rotary evaporation, and dried to obtain a yellow solid. After column chromatography (200-400 mesh silica gel), 1.76 g of yellow powder solid (6b) was obtained, with a yield of 61.9%.
[0040] After testing, 1 H NMR is: 1 H NMR (700MHz, CDCl 3 )δ7.75(d, J=15.6Hz, 1H), 7.67(dd, J=8.4, 1.8Hz, 1H), 7.61(d, J=1.8Hz, 1H), 7.51(d, J=15.6Hz, 1H), 7.27(d, J=5.8Hz, 1H), 7.19(d, J=7.7Hz, 1H), 7.16(s, 1H), 6.94(d...
Embodiment 3
[0045]
[0046] Preparation of (E)-3',5'-dihydroxy-3-methylchalcone (10a)
[0047] 1) Step 1: Synthesis of 3-hydroxy-5-methoxymethoxyacetophenone
[0048] Add 1.52g (0.01mol) of 3,5-dihydroxyacetophenone, 30mL of anhydrous acetone and 2.76g (0.02mol) of anhydrous potassium carbonate into a 50mL three-necked flask, stir in an ice bath for 30min, then slowly add 4mL (0.05 mol) bromomethyl methyl ether and 5mL of acetone mixed solution, reflux reaction for 4h. After the reaction was complete as monitored by TLC, the mixture was cooled to room temperature and impurities were filtered. After the solvent was evaporated to dryness by rotary evaporation, 1.58 g of oily liquid was obtained by silica gel column chromatography, with a yield of 65.8%.
[0049] 2) Step 2: Synthesis of (E)-3'-hydroxy-5'-methoxymethoxy-3-methylchalcone
[0050] Add 2.40g (0.01mol) of 3-hydroxy-5-methoxymethoxyacetophenone, 20mL of ethanol and 10mL of KOH (10%) aqueous solution in a 50mL three-necked fla...
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com