Novel chimeric antigen receptor
A technology of chimeric antigen receptors and receptors, which is applied in the field of biomedicine and can solve problems such as poor amplification ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
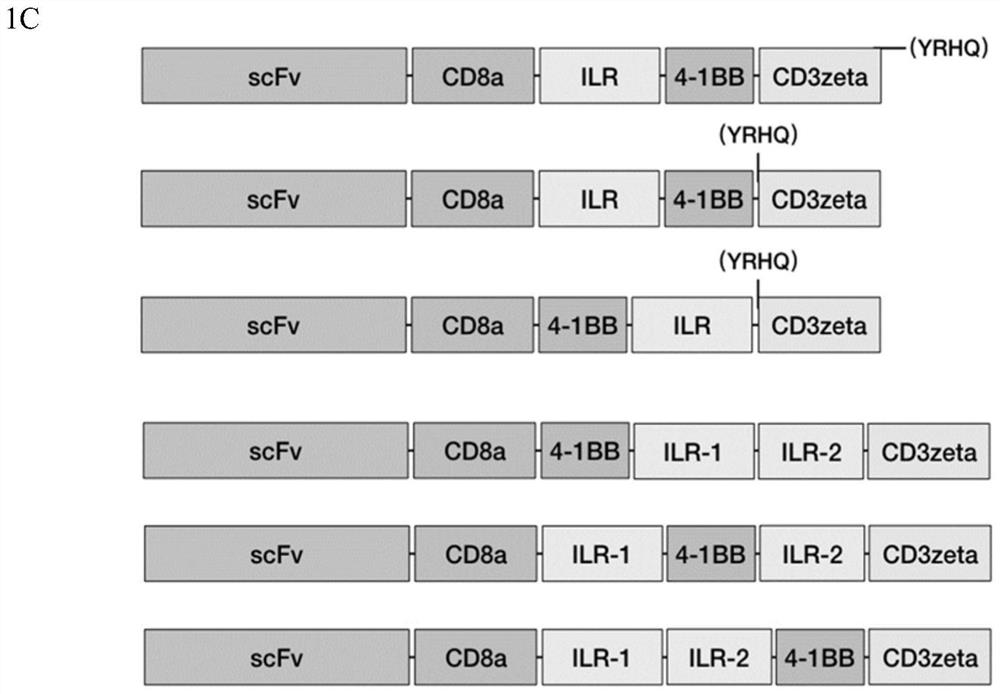
[0248] CLAIMS 1. A modified chimeric antigen receptor comprising at least one intracellular domain derived from a cytokine receptor.
[0249] 2. The modified chimeric antigen receptor of embodiment 1, wherein the intracellular domain is located in the intracellular portion of the modified chimeric antigen receptor.
[0250] 3. The modified chimeric antigen receptor of any one of embodiments 1-2, wherein the cytokine receptor comprises IL7RA, IL15RA, IL9R, IL3RA, IL21R and / or IL23R.
[0251] 4. The modified chimeric antigen receptor of any one of embodiments 1-3, wherein the cytokine receptor comprises IL7RA.
[0252] 5. The modified chimeric antigen receptor of any one of embodiments 1-4, wherein the cytokine receptor comprises IL21R.
[0253] 6. The modified chimeric antigen receptor of any one of embodiments 1-5, comprising a costimulatory domain.
[0254] 7. The modified chimeric antigen receptor of embodiment 6, wherein the costimulatory domain comprises a costimulatory ...
Embodiment 1
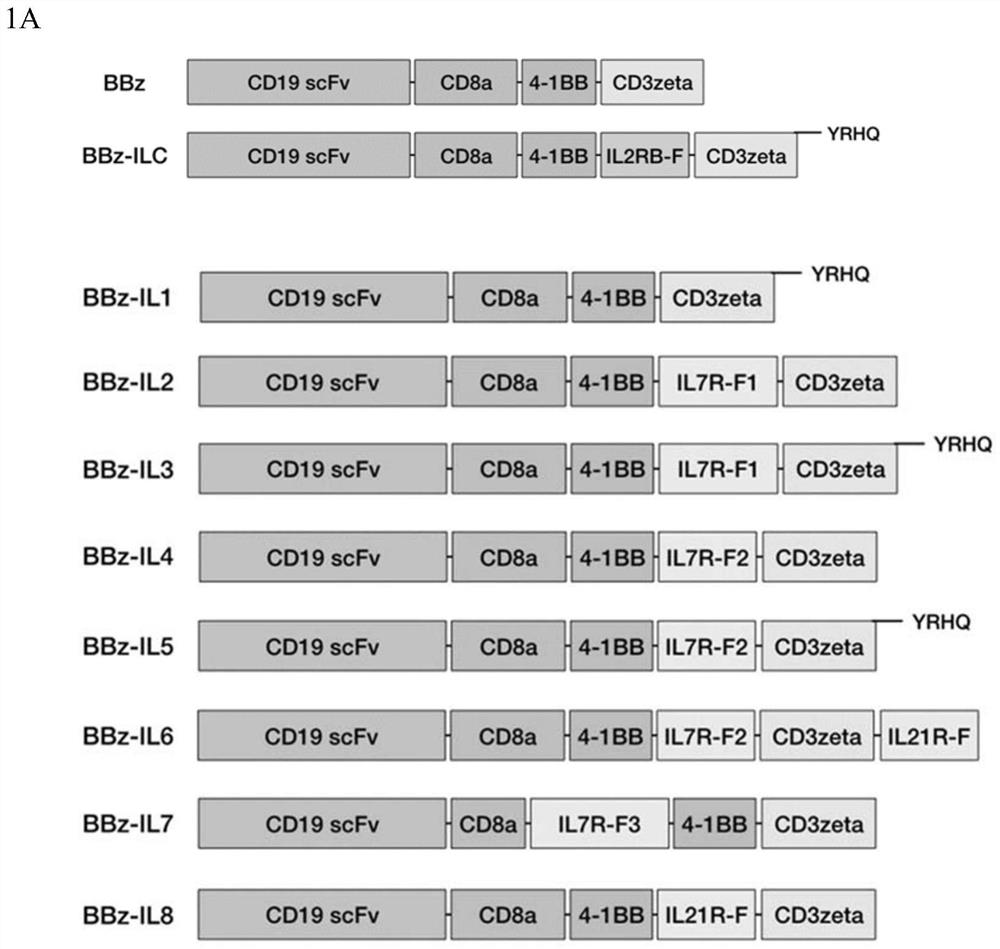
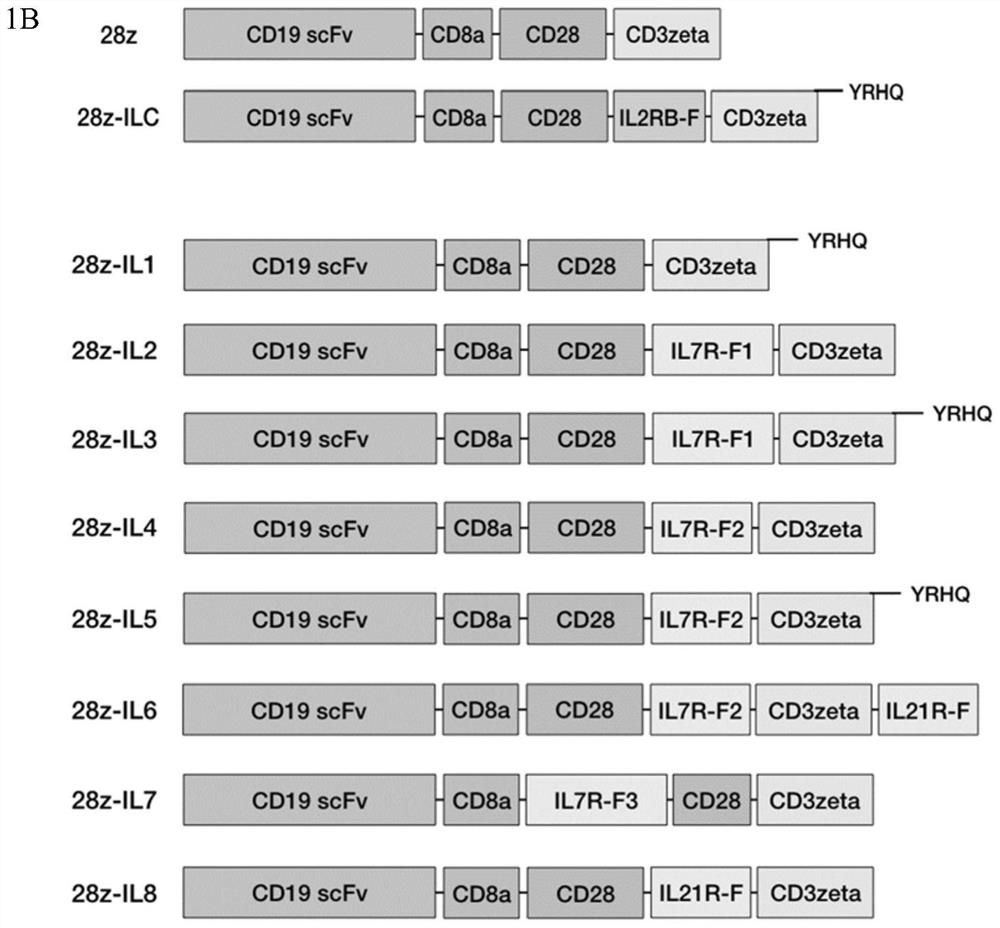
[0348] Example 1 Construction of a novel CAR structure
[0349] 1.1 Construction of BBz-ILR and 28z-ILR structures
[0350] 1.1.1 Nucleic acid sequences for synthesizing BBz and 28z
[0351] The nucleic acid sequences of BBz (SEQ ID NO: 1) and 28z (SEQ ID NO: 2) were obtained by gene synthesis. The corresponding amino acid sequences are BBz: SEQ ID NO:3, 28z: SEQ ID NO:4.
[0352] 1.1.2 Connect the nucleic acid sequences of BBz and 28z to the lentiviral vector pELPs
[0353] The BBz or 28z were ligated into the lentiviral vector pELPs (SEQ ID NO: 5) by restriction endonuclease BamHI digestion.
[0354] 1.1.3 Synthesis of ILR Sequence Fragments
[0355]The nucleic acid sequence of the truncated fragment (IL2RB-F) of the synthetic IL2RB intracellular domain (as shown in SEQ ID NO: 6, the amino acid sequence as shown in SEQ ID NO: 7), the nucleic acid sequence of YRHQ in BBz (SEQ ID NO: 61), the nucleic acid sequence (SEQ ID NO: 62) of YRHQ in 28z, the nucleic acid sequence ...
Embodiment 2
[0389] Example 2 Preparation of lentivirus with recombinant vector
[0390] 2.1 Extraction of recombinant vector
[0391] The pELPs-BBz, pELPs-28z, pELPs-BBz-ILC, pELPs-28z-ILC, pELPs-BBz-IL1 to 8 and pELPs-28z-IL1 to 8 recombinant vectors constructed above were retransformed into E. coli. Pick a single clone from the transformed plate and put it into a shaker tube of 3ml of liquid LB medium containing ampicillin, rotate at 220rpm, and cultivate with shaking on a shaker for 8h; draw 500μl from the activated bacterial solution and inoculate it into 250ml of ammonia-containing liquid In the liquid LB medium of penicillin, 220rpm, shaker for 12-16h. Recombinant vector extraction was performed using Qiagen HiSpeed Plasmid Maxi Kit (Cat. No. 12662) according to the experimental procedure provided by the kit. After extracting the recombinant vector, Nanodrop (Thermo Fisher Scientific) was used to detect the concentration of the recombinant vector, and the content of the recombin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com