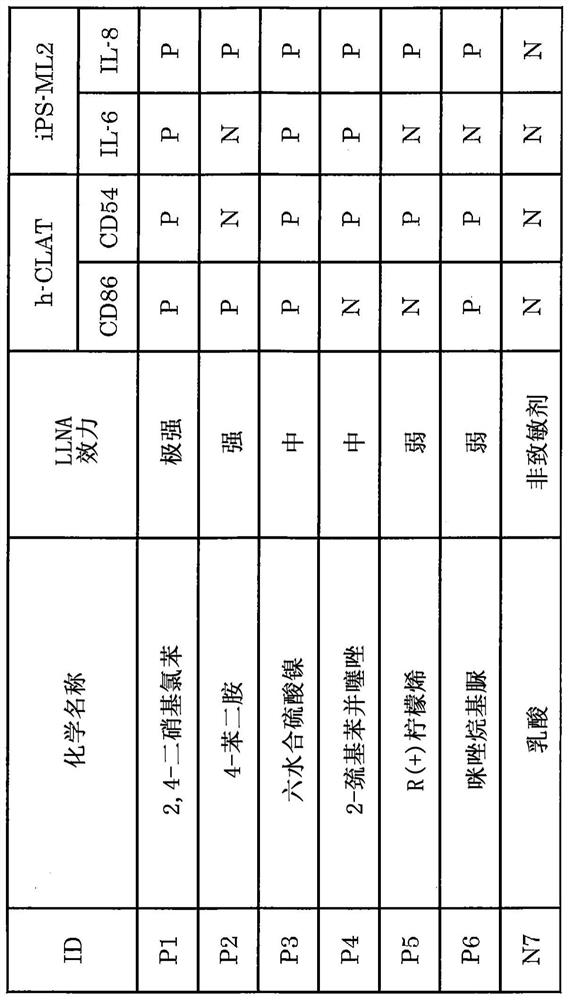
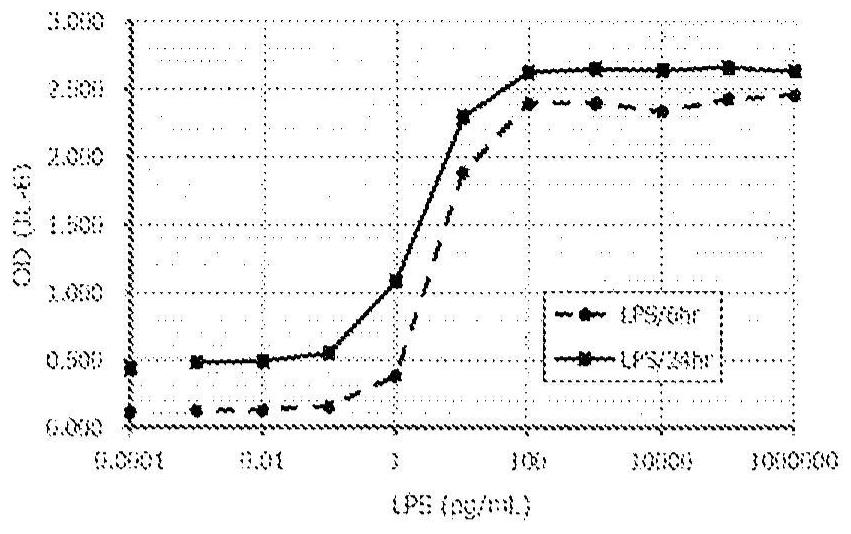
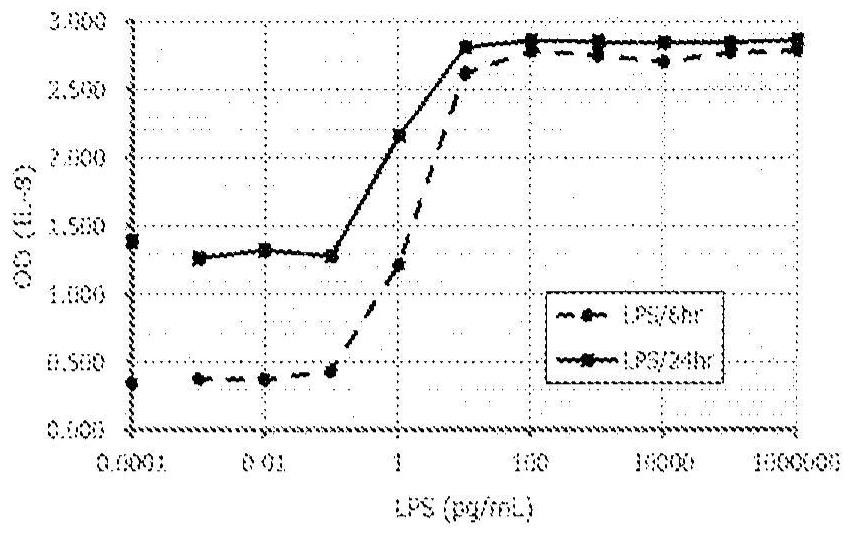
Method for in vitro evaluation of substance safety using human immortalized myeloid cells
An immortalization and material technology, applied in biochemical equipment and methods, microbial measurement/testing, biological testing, etc., can solve problems such as the absence of immune cells, difficulty in stable supply, individual differences, etc., and achieve precision and high sensitivity Evaluate and reduce the effect of error
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] (Example 1) Preparation of immortalized monocytes derived from human peripheral blood and preparation of dendritic cells
[0140] (Preparation of Immortalized Monocytes from Human Peripheral Blood)
[0141] Immortalized monocytes derived from human peripheral blood were prepared with reference to existing reports (WO2012 / 043651 and Japanese Patent Laid-Open No. 2017-131136). Specifically, CD14-positive fractions were extracted from human peripheral blood, and the genes (c-MYC, BMI1, and BCL-2) reported for the production of human immortalized monocytes were introduced into them according to existing reports, thereby producing immortalized cells. monocytes. Immortalized monocyte cell lines were grown in cells containing 20% FBS (Cytiva Inc., Cat#: SH30088.03), 50 ng / mL M-CSF (Peprotech Inc., Cat#: AF-300-25), and 50 ng / mL GM - Culture in α-MEM medium of CSF (Peprotech Inc., Cat#: 300-03), obtain proliferative cells 3 to 5 weeks after the start of culture, and use a c...
Embodiment 2
[0146] (Example 2) (Preparation of immortalized monocytes derived from human induced pluripotent stem cells)
[0147] Immortalized monocytes derived from human induced pluripotent stem (iPS) cells were prepared with reference to existing reports (WO2012 / 043651, JP 2017-131136 and JP 2018-171005). Specifically, undifferentiated iPS cells (provided by the iPS Cell Research Institute of Kyoto University) were seeded in 10 cm diameter petri dishes (AGC TECHNO GLASS CO., LTD., Cat#: 1012-100) or 6-well plates (Corning Inc., Cat#: 3516), culture dishes or 6-well plates were pre-coated with laminin 511 (iMatrix-511-E8, Nippi, Cat#: 892-012) as a basement membrane, and cultured in α-MEM. 20% FBS (Cytiva Inc., Cat#: SH30088.03) was added to the base (Fujifilm Wako Pure Chemical Industries, Ltd., Cat#: 137-17215) to obtain a culture medium (hereinafter referred to as α-MEM medium (containing 20 %FBS)), use this medium to start differentiation induction culture. After that, the α-MEM m...
Embodiment 3
[0150] (Example 3) Production of immortalized monocytes derived from human induced pluripotent stem cells expressing M-CSF and GM-CSF
[0151] Immortalized monocytes derived from human induced pluripotent stem cells expressing M-CSF and GM-CSF were prepared with reference to the existing report (Japanese Patent Laid-Open No. 2018-171005). Specifically, using a third-generation lentiviral vector (SignaGen Inc.) in which the promoter for expressing the protein is EF1a, the human induced pluripotent stem cell-derived immortalized monocytes obtained in Example 2 were simultaneously introduced with human M. - Expression vector of CSF gene and human GM-CSF gene, wherein the third-generation lentiviral vector is a strain of human immunodeficiency virus type 1 (HIV-I) lacking in proliferation ability and the like. 3 days after gene introduction, the cells were cultured in α-MEM (containing 20% FBS) medium that did not contain human M-CSF and GM-CSF, and 3 to 5 weeks after the start ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com