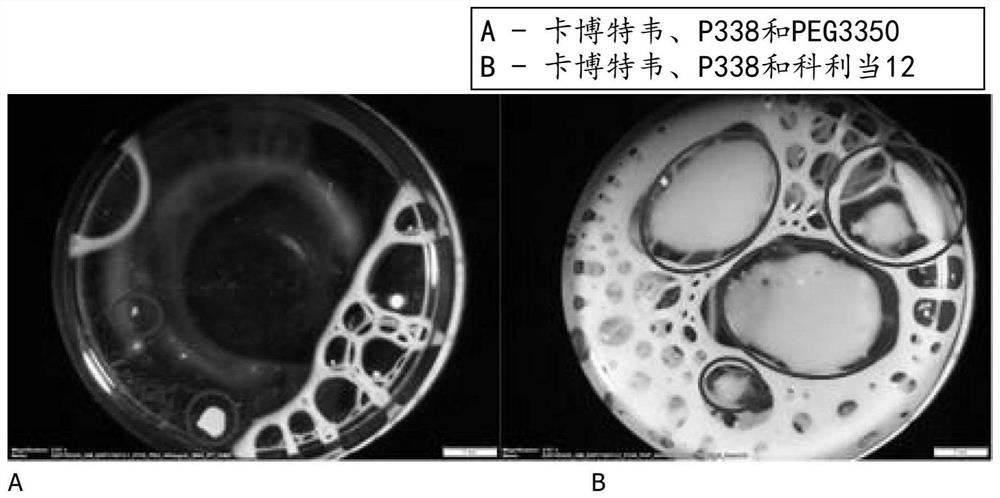
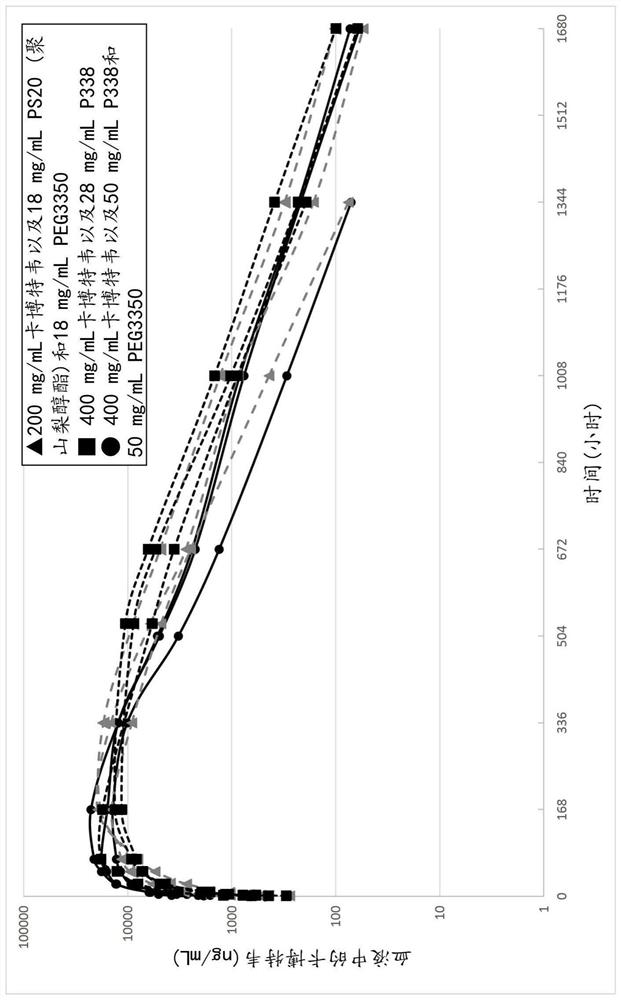
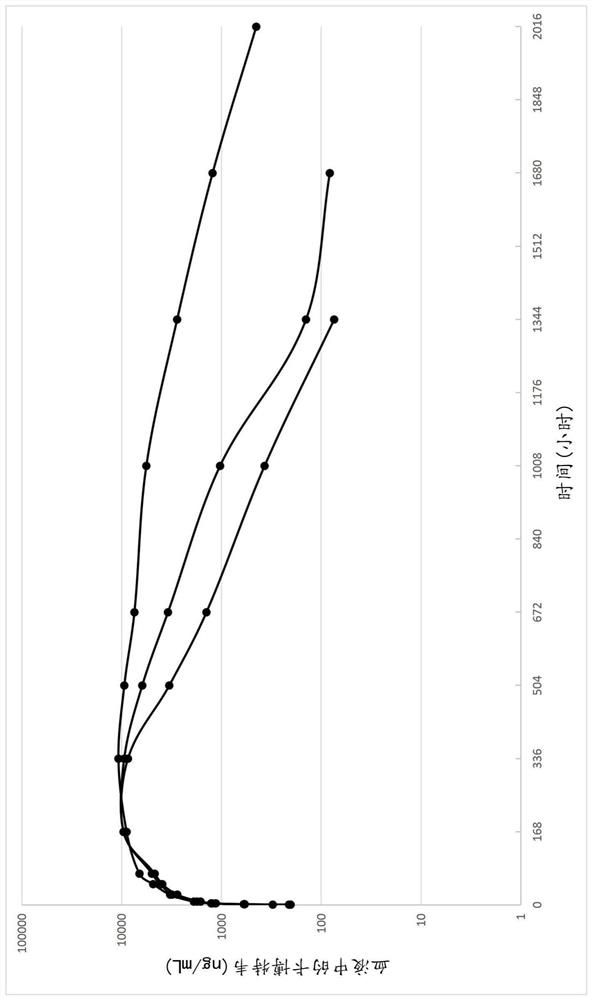
Pharmaceutical composition comprising cabotegravir
A technology of composition and injection composition, which is applied in the direction of drug delivery, active ingredients of heterocyclic compounds, active ingredients of hydroxyl compounds, etc., to achieve the effect of exhibiting particle size stability and realizing resuspension
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0265] Example 1: Composition of the invention
[0266]
[0267]
[0268] table 3
[0269] Table 3 shows exemplary pharmaceutical compositions of the invention (in these examples, the pharmaceutical compositions are also described as "suspensions"), prepared using the following method.
[0270]A formulation was prepared by dissolving 9.5 g of Poloxamer 338 (BASF), 5.0 g of mannitol (Roquette Freres) and 8.7 g of PEG3350 (Clariant) in 164.2 g of water for injection (WFI) and filtering the solution through a 0.2 μm filter vehicle. The formulation vehicle was added to cabotevir (free acid) to prepare a 400 mg / ml crude suspension. The crude suspension was circulated with stirring at 73-145 ml / min through a wet bead mill (Netzsch MiniCer) containing 0.30 mm YTZ beads (Nikato Corp) set at 29.7 Hz until reaching, as measured by laser diffraction, reached Desired median particle size of 0.2 to 1.0 μm. The wet bead mill was cooled to maintain a temperature between 1°C and 2...
Embodiment 2
[0271] Reality Example 2: Stability of Compositions Containing Cabotevir, Poloxamer 338 and PEG3350
[0272] A formulation was prepared by dissolving 7.5 g Poloxamer 338 (BASF), 5.7 g mannitol (Roquette Freres) and 7.5 g PEG3350 (Clariant) in 166.7 g water for injection (WFI) and filtering the solution through a 0.2 μm filter vehicle. The formulation vehicle was added to 100 g of cabotevir (free acid) to make a 400 mg / ml coarse suspension. The coarse suspension was circulated at 73-145 ml / min through a wet bead mill (Netzsch MiniCer) containing 0.30 mm YTZ beads (Nikato Corp) set at 29.7 Hz, while stirring, until the desired 0.25 μm was reached. median particle size. The wet bead mill was cooled to maintain a temperature between 1°C and 25°C. The milled suspension was filled into Type I glass vials, flushed with nitrogen, stoppered (FM457 stopper) and sealed. The resulting suspension was terminally sterilized by gamma irradiation at a minimum dose of 25 kGy. Table 4 sho...
Embodiment 3
[0282] Example 3: 400 mg / mL Cabotevir and Poloxamer 338 or 407
[0283] An injectable suspension containing about 400 mg / mL of cabotevir is prepared with Poloxamer 407 or 338. Mannitol was also added as a tonicity agent. The formulation vehicle was prepared by dissolving 7.0 g Poloxamer 338 and 3.5 g mannitol in 177.0 g WFI (Suspension 4), or 7.5 g P407 (BASF) and 4.7 g mannitol (Roquette Freres) was dissolved in 175.3 g of WFI (Suspension 3) and the solution was filtered through a 0.2 μm filter. The formulation vehicle was added to 100 g of cabotevir (free acid) to prepare a 400 mg / ml crude suspension. The crude suspension was circulated with stirring at 73-145 ml / min through a wet bead mill (Netzsch MiniCer) containing 0.30 mm YTZ beads (Nikato Corp) set at 29.7 Hz until the desired average of about 0.2 μm was reached particle size. The wet bead mill was cooled to maintain a temperature between 1°C and 25°C. The suspension was filled into Type I glass vials, flushed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com