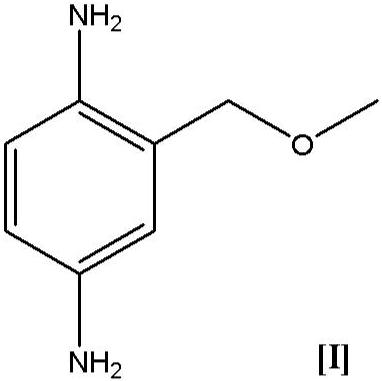
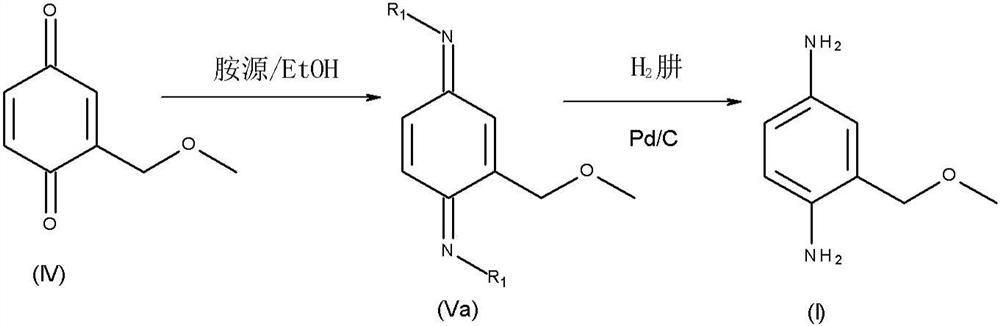
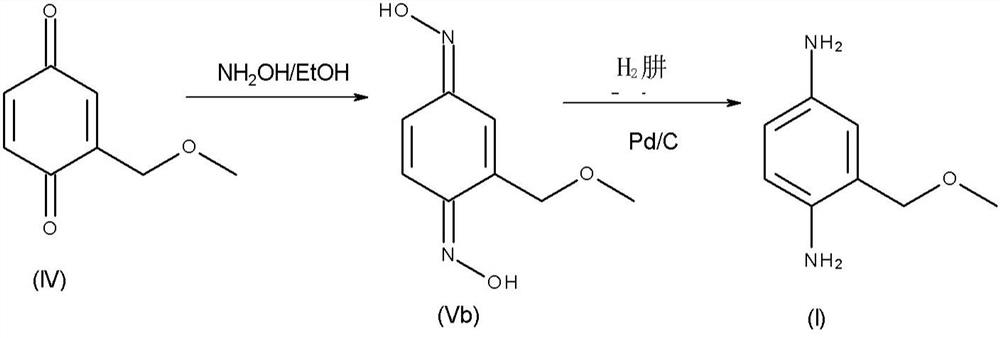
Novel shrinkage synthesis of 2-methoxymethyl-p-phenylenediamine
A technology of methoxymethyl and phenylenediamine, which is applied in the field of new-type condensation synthesis, and can solve the problems of negative impact on the appearance of powder materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0149] Example 1: Synthesis of 2-methoxymethyl-p-phenylenediamine (I) using 1,4-benzoquinone (VIII) as starting material
[0150] (1) Synthesis of 2-methoxymethyl-1,4-benzoquinone (IV)
[0151] A three-necked flask was fitted with a reflux condenser and thermometer and charged with 1 equiv = 1,41 g (8,24 mmol) of silver nitrate and a 1:1 mixture of dichloromethane and water (98 mL) in the presence of 0,3 equiv = 1,41 g (8,24 mmol). 3 g (27,5 mmol) of 1,4-benzoquinone (VIII), 1,5 equiv = 3,71 g (41,2 mmol) of 2-methoxyacetic acid (IX). The 2-phase mixture was stirred until complete dissolution was observed. The mixture was then heated to 40°C under reflux to float any sublimed 1,4-benzoquinone (VIII) back to the top of the flask. Ammonium peroxodisulfate (1,05 equiv = 6,72 g (28,8 mmol)) was then slowly added as a free radical former at a rate of 10 mL / h using a syringe driver or pump (dosimat) solution in water. The reaction mixture was kept at 40°C throughout the addition...
Embodiment 2
[0163] Example 2 Synthesis of 2-methoxymethyl-p-phenylenediamine (I) using 1,4-benzoquinone (VIII) as starting material
[0164] (1) Synthesis of 2-methoxymethyl-1,4-benzoquinone (IV)
[0165] A three-necked flask was fitted with a reflux condenser and thermometer and charged with 462 g of 1,4-benzoquinone ( VIII), 1,5 equivalents = 578 g of 2-methoxyacetic acid (IX). The 2-phase mixture was stirred until complete dissolution was observed. The mixture was then heated to 39°C under reflux to float any sublimed 1,4-benzoquinone (VIII) back to the top of the flask. Then a solution of 1073 g of ammonium peroxodisulfate as a free radical former in 2080 mL of water was slowly added over 1 hour. The reaction mixture was kept at 40°C throughout the addition. After the addition was complete, the mixture was stirred at 39°C under reflux for an additional 2 hours. HPLC analysis showed 60% conversion from benzoquinone (VIII) to the desired intermediate 2-methoxymethyl-1,4-benzoquinon...
Embodiment 3
[0177] Example 3: Synthesis of 2-methoxymethyl-p-phenylenediamine (I) and p-phenylenediamine (XI) as a by-product
[0178] (1) Synthesis of 2-methoxymethyl-1,4-benzoquinone (IV)
[0179] A three-necked flask was fitted with a reflux condenser and thermometer and charged with 1 equiv = 3 g (27,5 mmol) of 1 in the presence of 0,3 equiv = 1,41 g (8,24 mmol) of silver nitrate and water (98 mL). 4-Benzoquinone (VIII), 1,5 equiv = 3,71 g (41,2 mmol) of 2-methoxyacetic acid (IX). The mixture was stirred and heated to 65°C until complete dissolution was observed. The aqueous phase was continuously floated with a pump to avoid sublimation of 1,4-benzoquinone (VIII) at the top of the flask. Ammonium peroxodisulfate (1,05 equiv = 6,72 g (28,8 mmol)) was then slowly added as a free radical former at a rate of 10 mL / h using a syringe driver or pump (dosimat) solution in water. The reaction mixture was maintained at 65°C throughout the addition. After the addition was complete, the mix...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com