Method for preparing thiolactide
A technology of thiolactide and cyclic thiolactide, which is applied in the field of preparation of thiolactide, can solve the problems of time-consuming, unsuitable for large-scale production, and many waste by-products, so as to save resources and costs, and is an important application value, avoidance of process effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
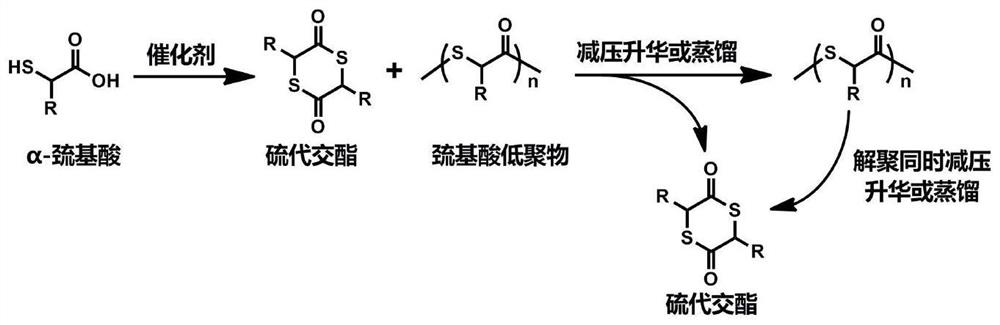
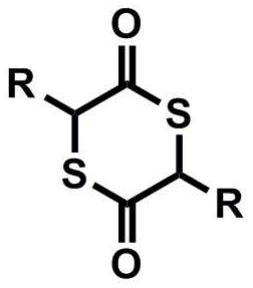
[0045] Weigh 92g of thioglycolic acid into a 2L round-bottom flask, add 1L of toluene to dissolve, then slowly add 7.5g (4.0ml) of trifluoromethanesulfonic acid, and react at 120°C with water for 6h. The reaction is stopped, and the toluene is spin-dried to obtain a mixture of cyclic thioglycolide and thioglycolic acid oligomer, and the steamed toluene can be recovered and reused.
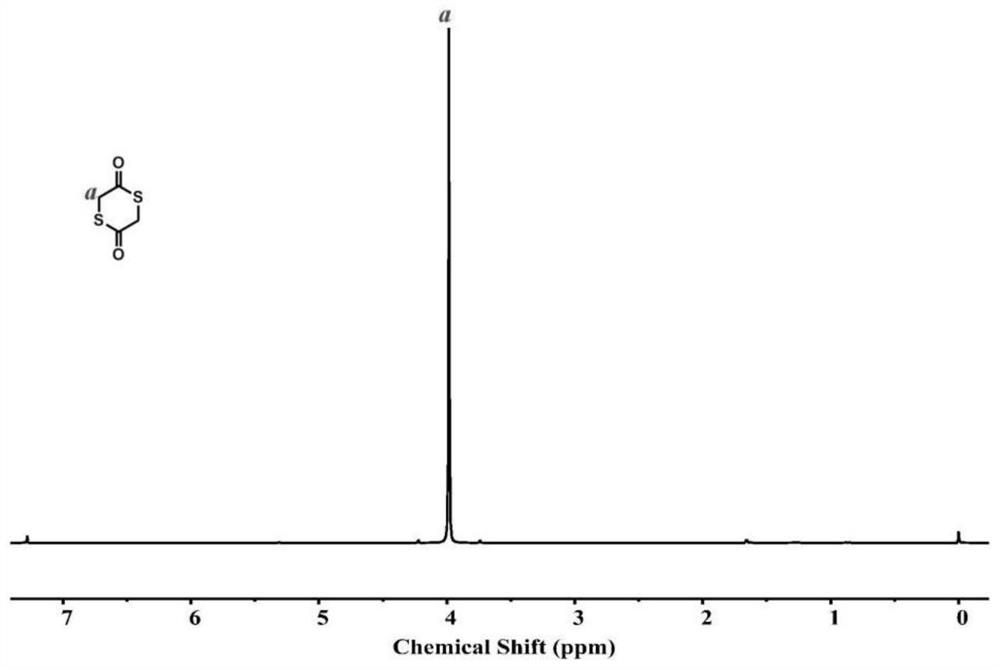
[0046] The above mixture was transferred to a vacuum sublimation device, and sublimed under reduced pressure at 120° C. and a vacuum degree of about 100 Pa, and the product was collected, which was directly cyclic thioglycolide. Add 3g sodium thiophenate and 30g polyethylene glycol monomethyl ether to the remaining product, continue to sublime under reduced pressure, and collect the sublimation product, which is the thioglycolide after depolymerization, and the cosolvent polyethylene glycol monomethyl ether. Can be recycled and reused. A total of 56g was obtained by two sublimations (the yield was...
Embodiment 2
[0048] Weigh 92g of thioglycolic acid into a 2L round-bottom flask, add 1L of toluene to dissolve, then add 17g of tetrabutyl titanate, and react at 120°C with water for 8h. The reaction is stopped, and the toluene is spin-dried to obtain a mixture of cyclic thioglycolide and thioglycolic acid oligomer, and the steamed toluene can be recovered and reused.
[0049] The above mixture was transferred to a vacuum sublimation device, and sublimed under reduced pressure at 120° C. and a vacuum degree of about 100 Pa, and the product was collected, which was directly cyclic thioglycolide. Add 2.5g sodium thiophenate and 30g polyethylene glycol monomethyl ether to the remaining product, continue to sublime under reduced pressure, collect the sublimation product, namely the thioglycolide after depolymerization, and the cosolvent polyethylene glycol monomethyl ether. Ether can be recycled and reused. A total of 48 g were obtained by two sublimations (yield about 65%, purity 94%), 1 H ...
Embodiment 3
[0051] Weigh 92g of thioglycolic acid into a 2L round-bottom flask, add 1L of toluene to dissolve, then add 16.7g of pentafluoroaniline trifluoromethanesulfonate, and react at 80°C for 8h. The reaction is stopped, and the toluene is spin-dried to obtain a mixture of cyclic thioglycolide and thioglycolic acid oligomer, and the steamed toluene can be recovered and reused.
[0052] The above mixture was transferred to a vacuum sublimation device, and sublimed under reduced pressure at 120° C. and a vacuum degree of about 100 Pa, and the product was collected, which was directly cyclic thioglycolide. Add 3.2g of sodium thiophenate and 30g of polyethylene glycol monomethyl ether to the remaining product, continue to sublime under reduced pressure, and collect the sublimation product, which is the thioglycolide after depolymerization, and the cosolvent polyethylene glycol monomethyl ether. Ether can be recycled and reused. A total of 53g was obtained by two sublimations (yield abou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com