Method for determining imidacloprid, sulfluramid and metabolites thereof
A technology of imidacloprid and sulfluramid, which is applied in the field of analytical chemistry, can solve the problems of time-consuming and laborious, large pre-treatment sampling amount, and many extraction solvents, etc., and achieves the effects of simple pre-treatment, small sampling amount, and low usage amount.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
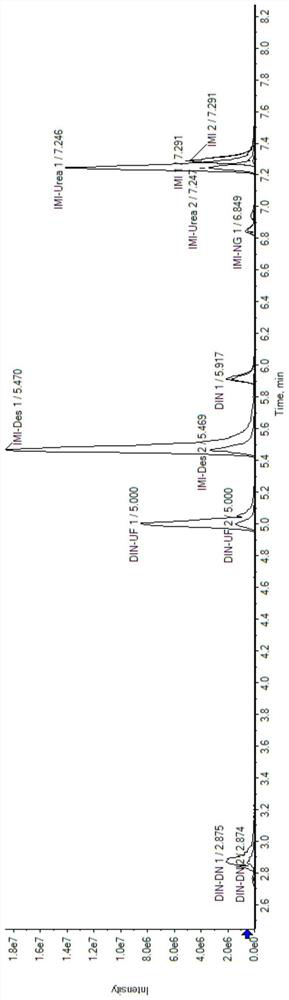
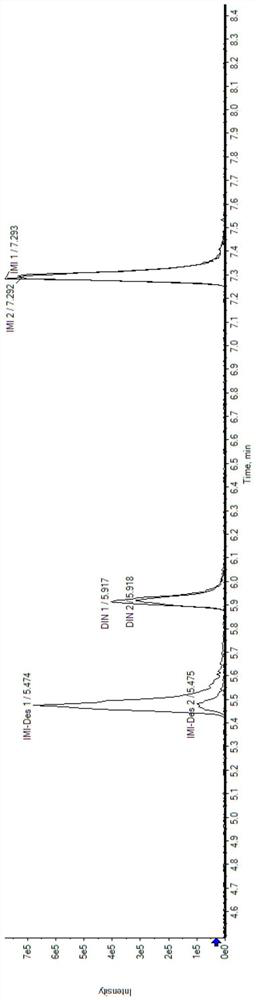
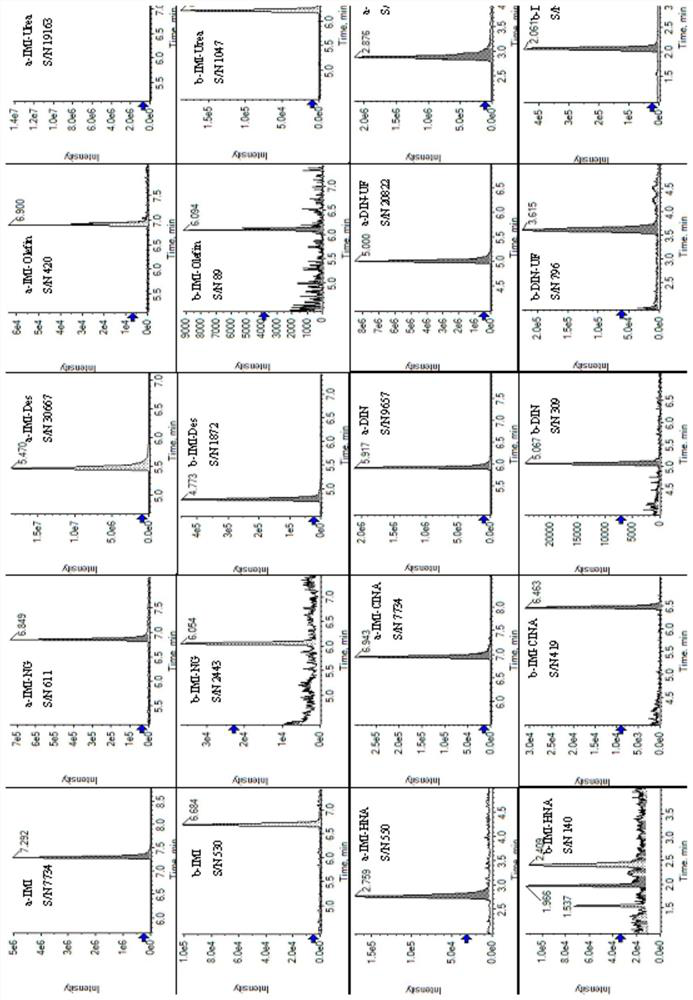
[0052] Example 1: Determination of imidacloprid and sulfluramid and their metabolites in soil
[0053] 1. Sample pretreatment
[0054] Preparation of extraction solvent: acetonitrile: water ≈ 1:1 (v / v), plus 5% formic acid;
[0055] Soil extraction: Weigh 0.2 g of freeze-dried soil, add 400 μL of extraction solvent, vortex for 1 min, shake for 10 min, centrifuge for 10 min, and take 100 μL of supernatant to obtain soil extraction samples. Add 100 μL of pure water, mix well and centrifuge for 10 min, take the supernatant to obtain a liquid sample for loading, and perform LC-MS / MS detection.
[0056] 2. LC-MS / MS detection
[0057] Instrument: ExionLC TM AD+QTRAP 6500 plus
[0058] liquid phase method
[0059] Chromatographic column: Agilent XBD C18 (100mm*3mm, 1.8μm);
[0060] Column temperature: 40℃;
[0061] Injection volume: 1 μL;
[0062] Aqueous phase A: an aqueous solution containing 2 mM ammonium formate and 0.01% formic acid;
[0063] Organic phase B: methanol ...
Embodiment 2
[0085] Example 2: Detection of matrix effects
[0086] Using soil without pesticide residues as raw material, prepare matrix solvent as follows: Weigh 0.2 g of lyophilized soil, add 400 μL of extraction solvent (acetonitrile: water = 1:1 v / v, containing about 5% formic acid), vortex Spin for 1 min, shake for 10 min, centrifuge for 10 min, take the supernatant, add the same amount of pure water, mix well, centrifuge for 10 min, take the supernatant to obtain the matrix solvent.
[0087] The blank solvent was prepared according to the following method: acetonitrile:water=1:3 (v / v) solution was the blank solvent.
[0088] The above solvents were used to prepare the concentrations of each compound to be 0.01ng / mL, 0.05ng / mL, 0.1ng / mL, 0.5ng / mL, 1ng / mL, 5ng / mL, 10ng / mL, 50ng / mL, 100ng / mL, 500ng / mL and 1000ng / mL matrix standard and solvent standard, enter LC-MS / MS detection, record the peak area, fit the matrix standard curve and solvent standard curve, divide the slope of the matr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com