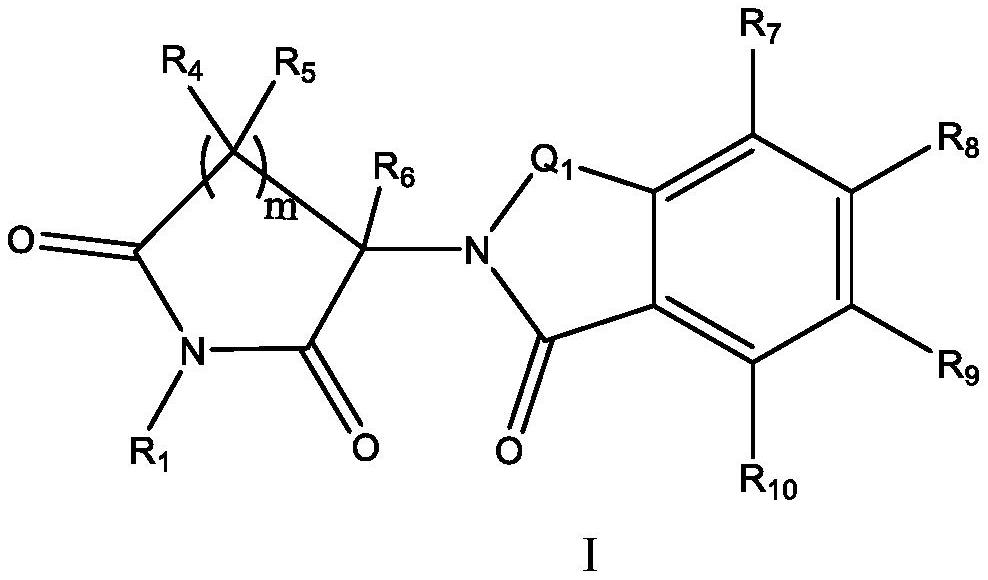
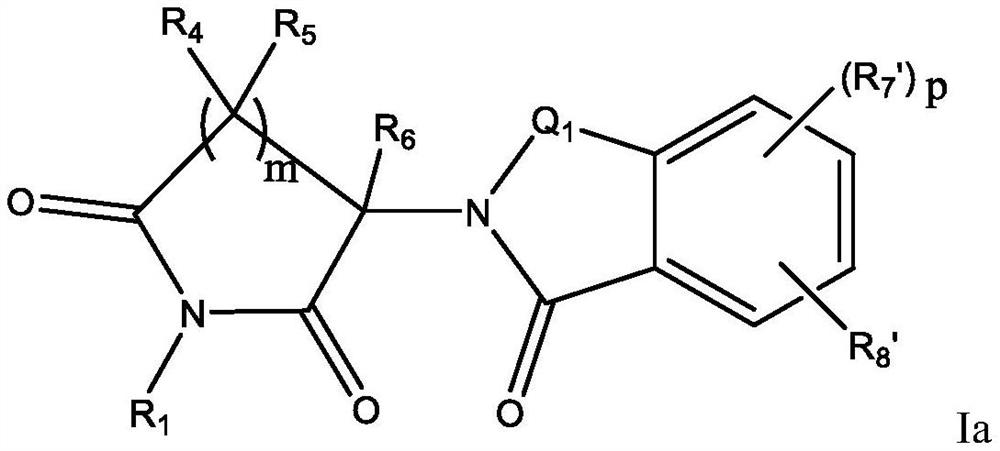
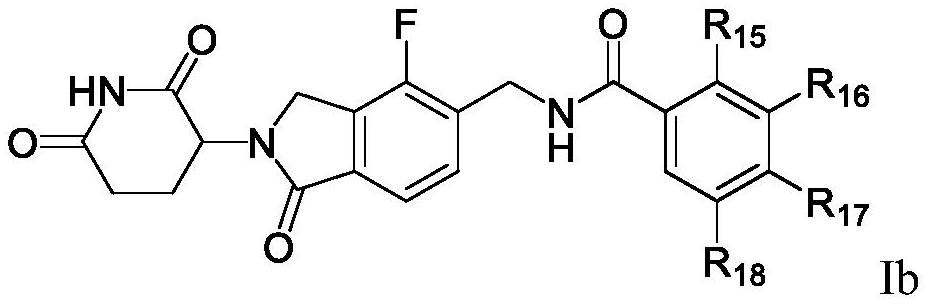
Halogen-substituted isoindoline compound and application thereof
A compound and halogen technology, applied in the field of medicine, can solve the problem of halogen-substituted isoindoline compounds and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Synthesis of compound FDAB-1
[0089]
[0090] Synthesis of T1-1: Cuprous cyanide (5.28 g, 59.0 mmol) and tert-butyl nitrite (14 g, 136.1 mmol) were dissolved in 100 ml of dimethyl sulfoxide, and the temperature was raised and kept at 60 °C. After 30 minutes, a solution of 4-bromo-3-fluoro-2-methylaniline (10 g, 49.0 mmol) in dimethyl sulfoxide (20 mL) was slowly added dropwise for about 30 minutes. After stirring the reaction at 60°C for about 1 hour, LCMS showed that the starting material had disappeared. The reaction solution was cooled to room temperature, 6M hydrochloric acid solution was added dropwise to the reaction solution to quench the reaction, and then ethyl acetate (2×100 mL) was added for extraction and separation. The organic phases were combined and backwashed with saturated brine. The organic phase was dried over anhydrous sodium sulfate, filtered, and concentrated under reduced pressure to obtain the crude product. After column chromat...
Embodiment 2
[0096] Example 2: Synthesis of compound FDAB-2
[0097] Dissolve FDAB-1 (5.7 g, 20.0 mmol) and di-tert-butyl dicarbonate (6.5 g, 30.0 mmol) in 60 mL of tetrahydrofuran / N,N-dimethylformamide=10:1 mixed solvent at room temperature Raney nickel (0.6 g) was added. The mixed system was replaced by hydrogen three times while maintaining a certain hydrogen pressure system. The temperature was raised to 40° C. and the reaction was stirred at this temperature for about 4 hours. LCMS detection showed that the reaction of FDAB-1 was complete. The reaction solution was cooled and filtered with suction, the filter cake was washed three times with 50 ml of dichloromethane / methanol (10 / 1), the filtrates were combined and concentrated under reduced pressure. Then, 100 mL of water was added to the concentrated solution, followed by extraction and separation with ethyl acetate (3×100 mL). The organic phases were combined and backwashed with saturated brine. The organic phase was dried over an...
Embodiment 3
[0098] Example 3: Synthesis of compound FDAB-3
[0099] The intermediate FDAB-2 (3.9 g, 10 mmol) was dissolved in 30 mL of dichloromethane, and a solution of hydrochloric acid in 1,4-dioxane (1 M, 5 mL) was added at room temperature and the temperature was raised to 40 °C for reaction. After about 1 hour of reaction, LCMS detection showed that the raw material FDAB-2 disappeared. Concentrate under reduced pressure to remove excess hydrochloric acid solution and solvent, adjust the concentration to dichloromethane and adjust the acidity to ~7 with ammonia water, concentrate again and perform column chromatography (methanol: dichloromethane=1 / 100-1 / 10) to obtain off-white solid product FDAB-3 is about 2.8 g, and the yield is about 86%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com