Metallographic corrosive agent suitable for multi-component aluminum alloy and corrosion method of metallographic corrosive agent
A metallographic corrosion and aluminum alloy technology, applied in the field of multi-component aluminum alloy metallographic etchant and its corrosion, can solve the problems of small application range, unclear metallographic structure, affecting experimental efficiency, etc., and achieve a wide range of corrosion time , grain boundaries are clean and clear, and the effect of a wide range of applications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
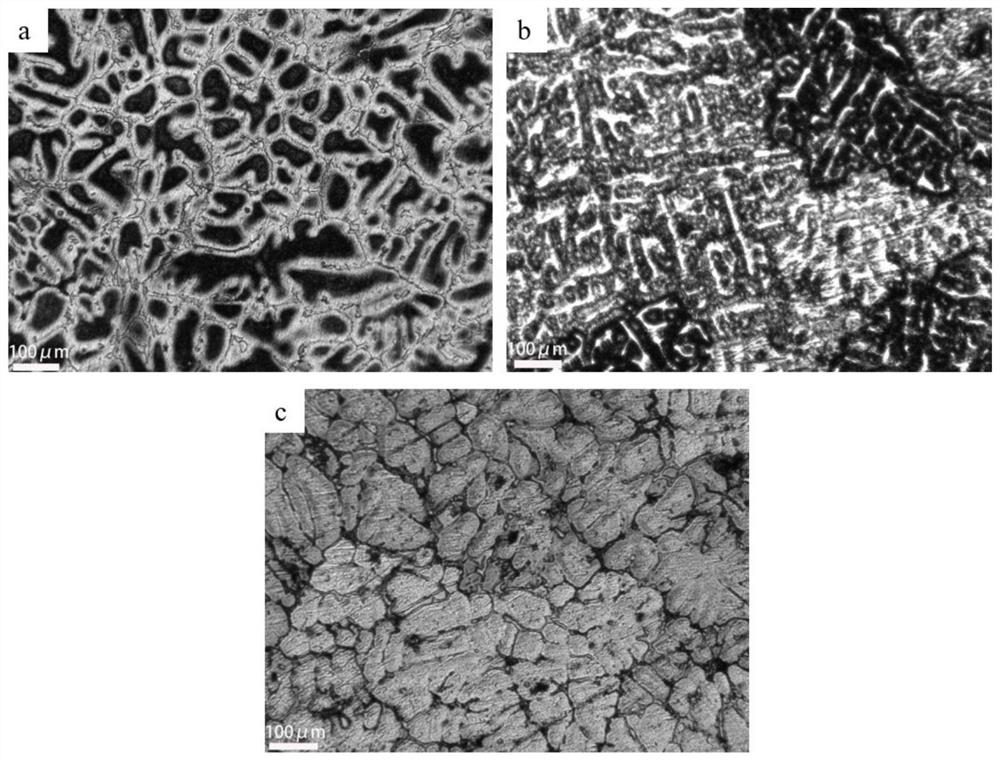
[0041] A method for preparing a 2024-Ni-Fe-Mn metallographic sample, comprising:
[0042] (1) Weigh 2g of NaF with an electronic balance, measure 5ml of HCl with a concentration of 36% in a measuring cylinder, pour the two medicines into the test tube and fully react until the NaF is completely dissolved. After the dissolution is complete, pour the solution into a 90ml H 2 O beaker and stir with a glass rod. Measure 2ml of HNO with a concentration of 66% with a graduated cylinder in turn 3 and 3ml CH 3 OH, pour it into the previous beaker, stir with a glass rod, and dissolve it fully;
[0043] (2) The aluminum alloy sample is polished with metallographic sandpaper and polished to a mirror surface;
[0044] (3) Use a cotton swab or a rubber tip dropper to take a small amount of the corrosive agent in the beaker and spread it evenly on the surface of the sample until the liquid completely covers the aluminum alloy mirror surface;
[0045] (4) Start timing when the etchant c...
Embodiment 2
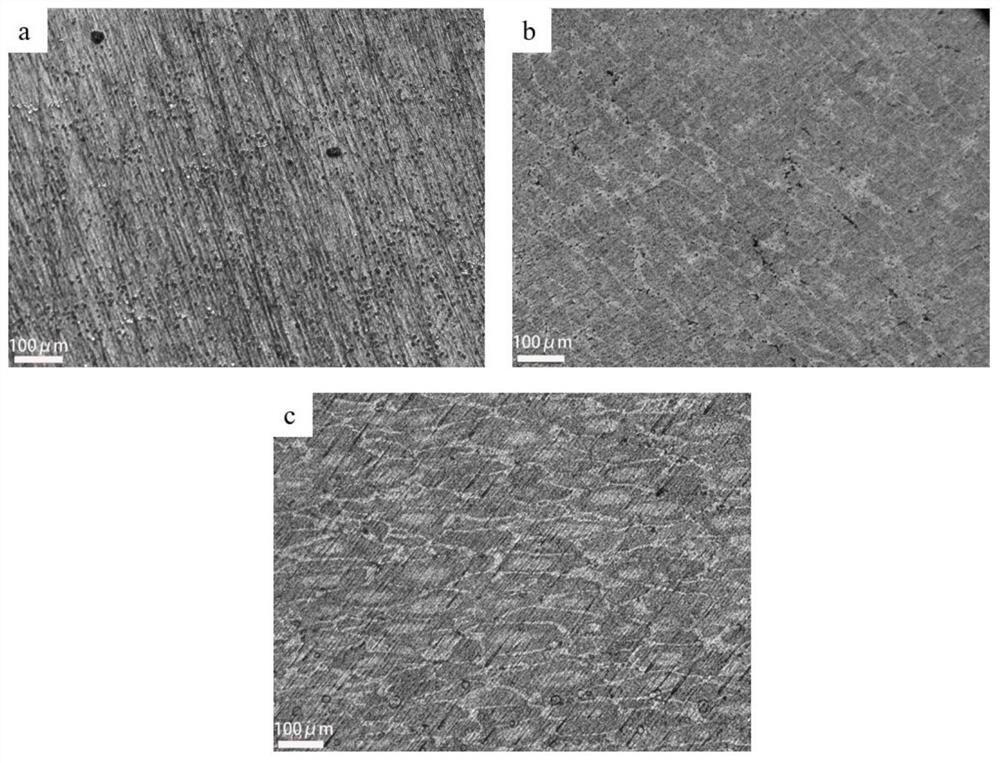
[0052] A method for preparing a 7085-Sc-Mn metallographic sample, comprising:
[0053] (1) Weigh 1g of NaF with an electronic balance, measure 5ml of HCl with a concentration of 36% in a measuring cylinder, pour the two medicines into the test tube and fully react until the NaF is completely dissolved. After the dissolution is complete, pour the solution into a 89ml H 2 O beaker and stir with a glass rod. Measure 2ml of HNO with a concentration of 66% with a graduated cylinder in turn 3 and 3ml CH 3 OH, pour it into the previous beaker, stir with a glass rod, and dissolve it fully;
[0054] (2) The aluminum alloy sample is polished with metallographic sandpaper and polished to a mirror surface;
[0055] (3) Use a cotton swab or a rubber tip dropper to take a small amount of the corrosive agent in the beaker and spread it evenly on the surface of the sample until the liquid completely covers the aluminum alloy mirror surface;
[0056] (4) Start timing when the etchant cont...
Embodiment 3
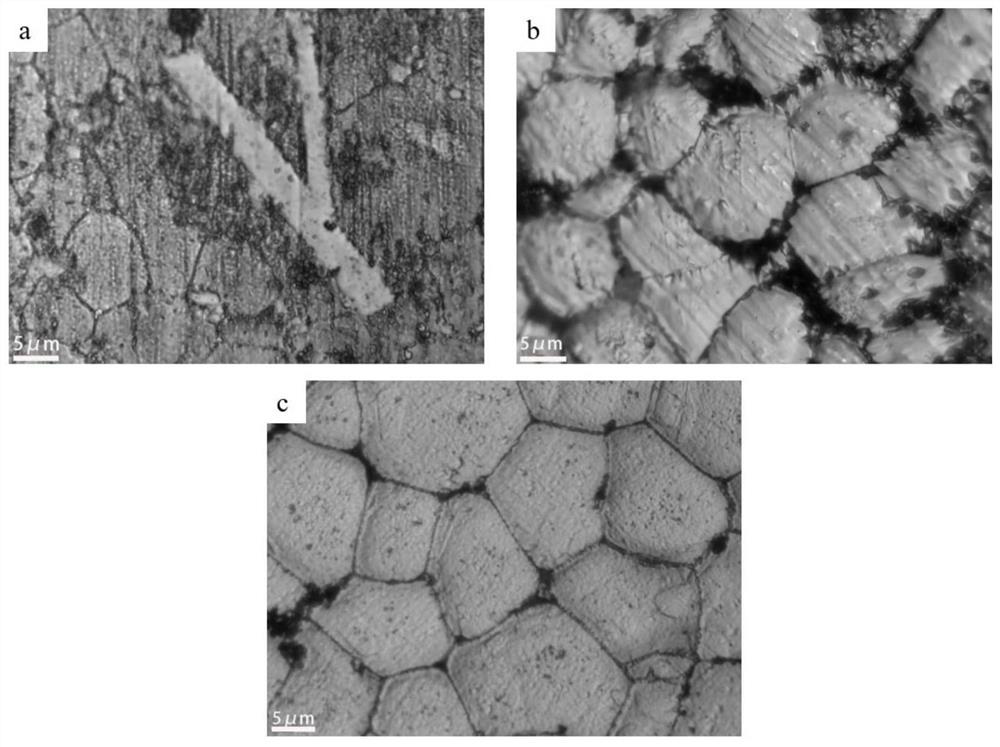
[0063] A method for preparing the metallographic phase of 7085-Sc-Er-ISNCP aluminum alloy, comprising:
[0064] (1) Weigh 0.5g of NaF with an electronic balance, measure 5ml of HCl with a concentration of 36% in a measuring cylinder, pour the two drugs into the test tube and fully react until the NaF is completely dissolved. After the dissolution is complete, pour the solution into a Has 90ml H 2 O beaker and stir with a glass rod. Measure 2ml of HNO with a concentration of 66% with a graduated cylinder in turn 3 and 3ml CH 3 OH, pour it into the previous beaker, stir with a glass rod, and dissolve it fully;
[0065] (2) The aluminum alloy sample is polished with metallographic sandpaper and polished to a mirror surface;
[0066] (3) Use a cotton swab or a rubber tip dropper to take a small amount of the corrosive agent in the beaker and spread it evenly on the surface of the sample until the liquid completely covers the aluminum alloy mirror surface;
[0067] (4) Start t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com