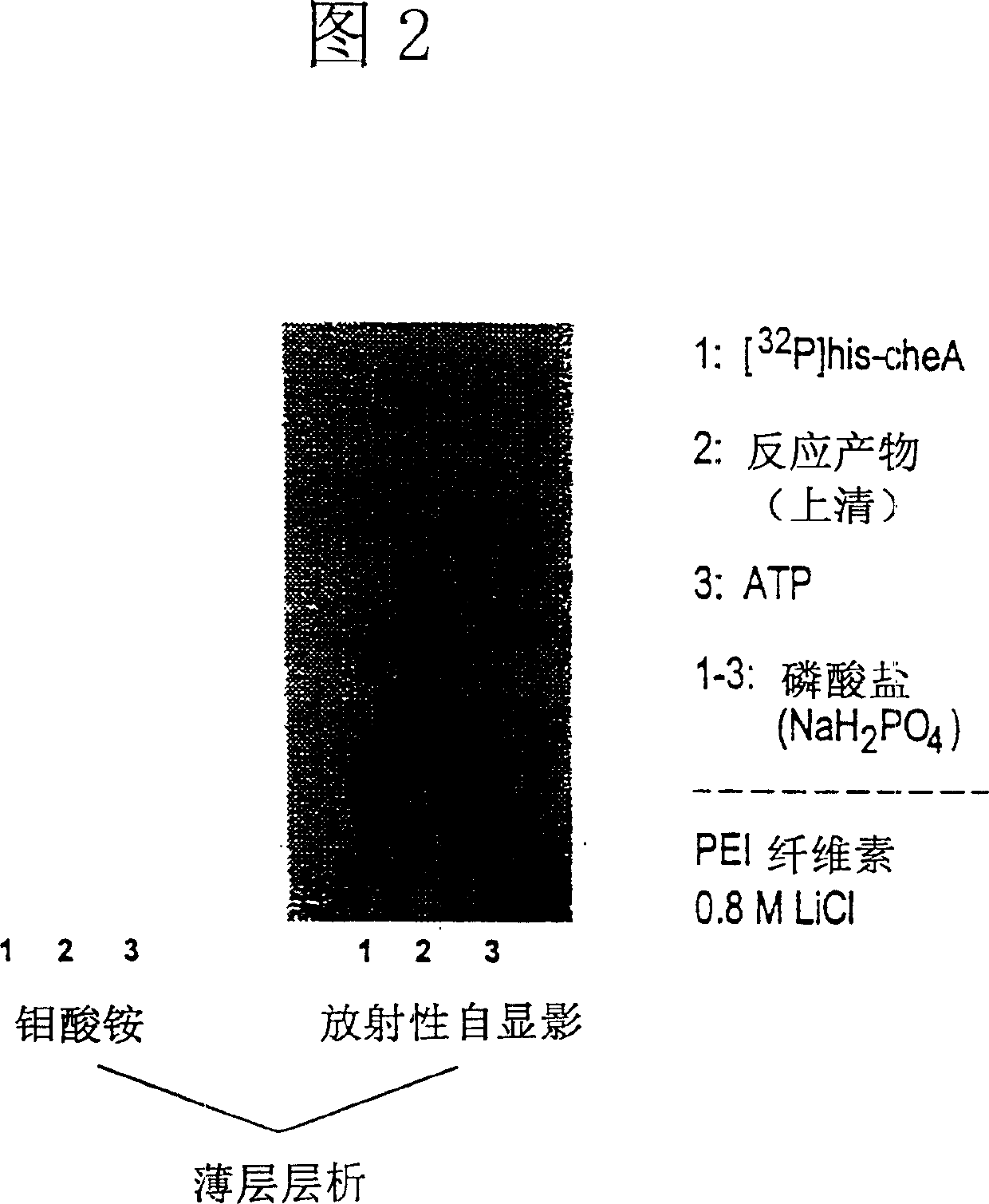
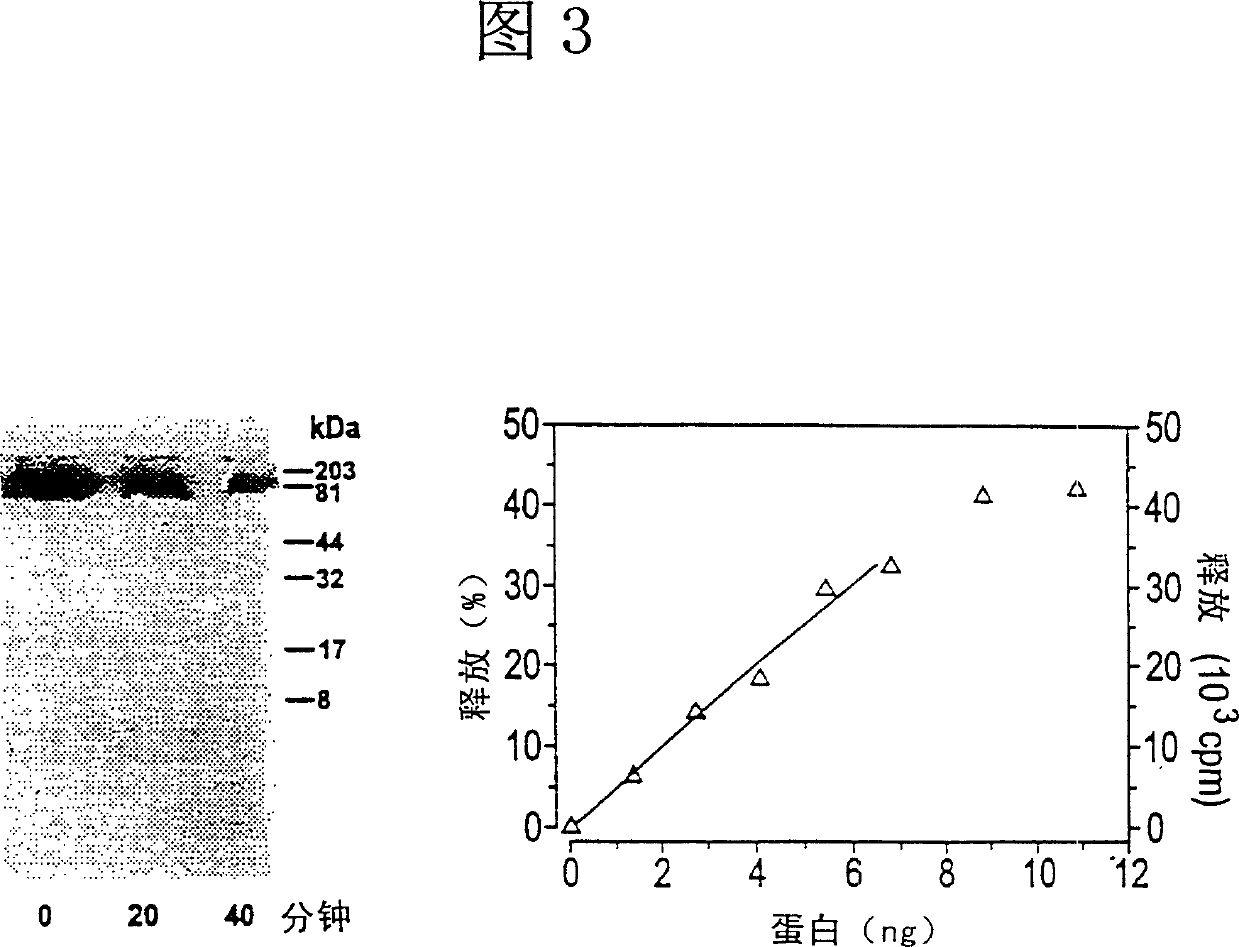
Histidine protein-phosphatase
A technology of histidine phosphatase and phosphohistidine, which is applied to peptide/protein components, anti-enzyme immunoglobulin, hydrolase, etc., can solve the problems of undetectable and unstable hydrolysis of histidine phosphatase.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0039] (A) Separation and purification
[0040] Histidine protein phosphatase was isolated from rabbit liver.
[0041] About 110 grams of rabbit liver samples were cut into small pieces, mixed with homogenization buffer (220 ml 30 mM triethanolamine / hydrochloric acid pH 7.5, 1 mM ethylenediamine tetraacetic acid, 300 mM sucrose, 0.1 mM benzamidine, 0.1% 2-mercaptoethanol ) and grind in a homogenizer cooled on ice. After the first centrifugation (10 minutes at 3800g, 4°C), the supernatant was centrifuged again (1 hour at 48000g, 4°C). The supernatant was filtered through gauze and frozen at -80°C in approximately 20 ml aliquots.
[0042] Column chromatography separation method
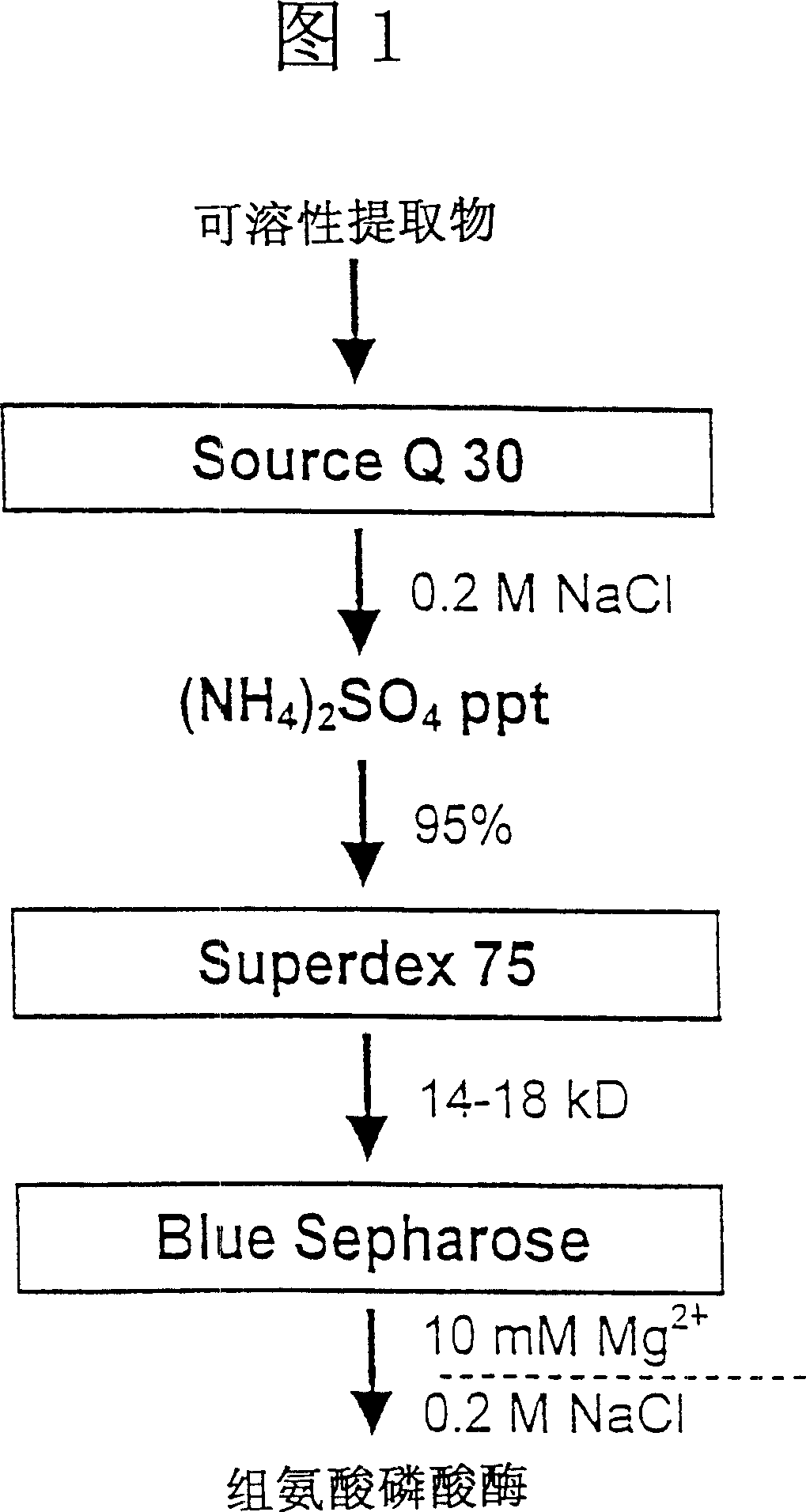
[0043] The histidine protease was isolated using a three-step purification procedure (Figure 1).
[0044] 1. Anion exchange chromatography
[0045] The crude liver extract was centrifuged again (48000 g for 30 minutes). Add the supernatant to a Source Q30 (Pharmacia, Freiburg) column equilibrat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com