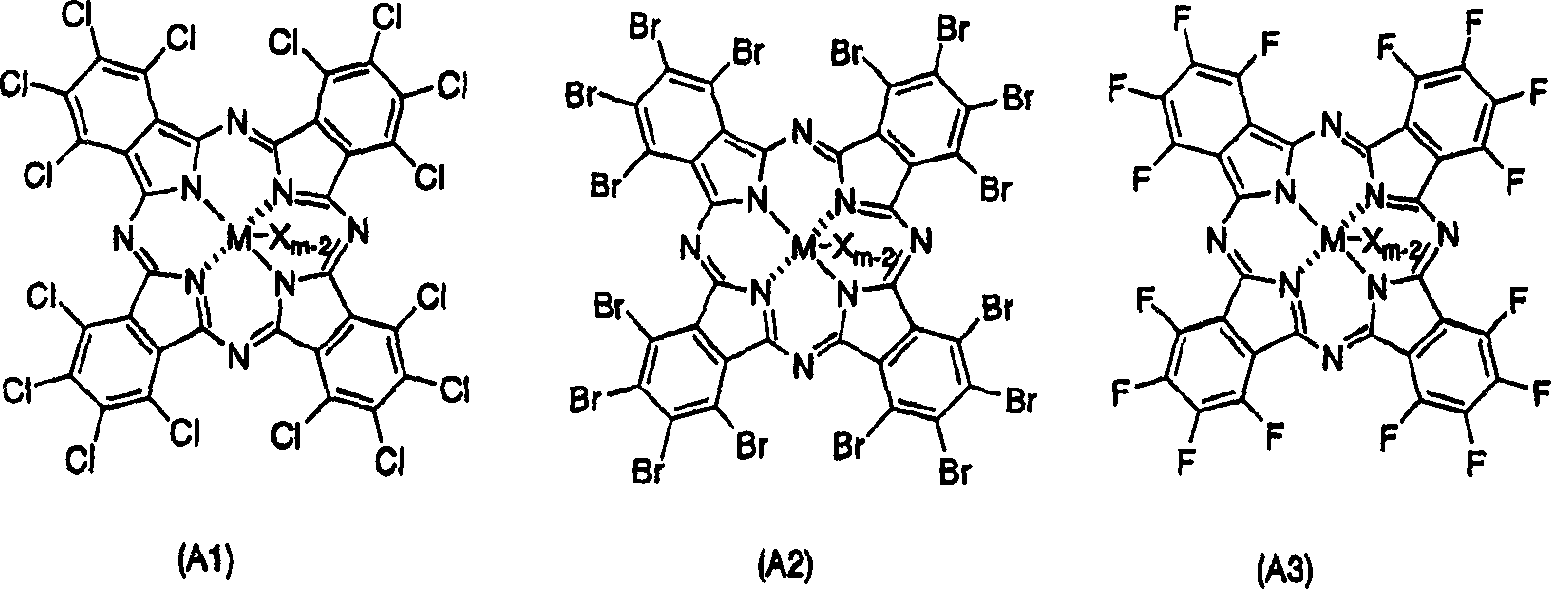
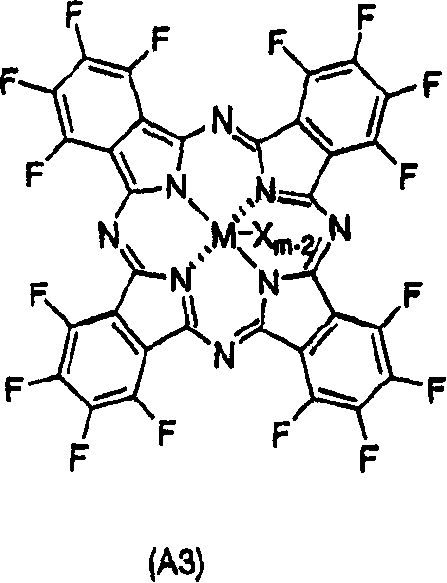
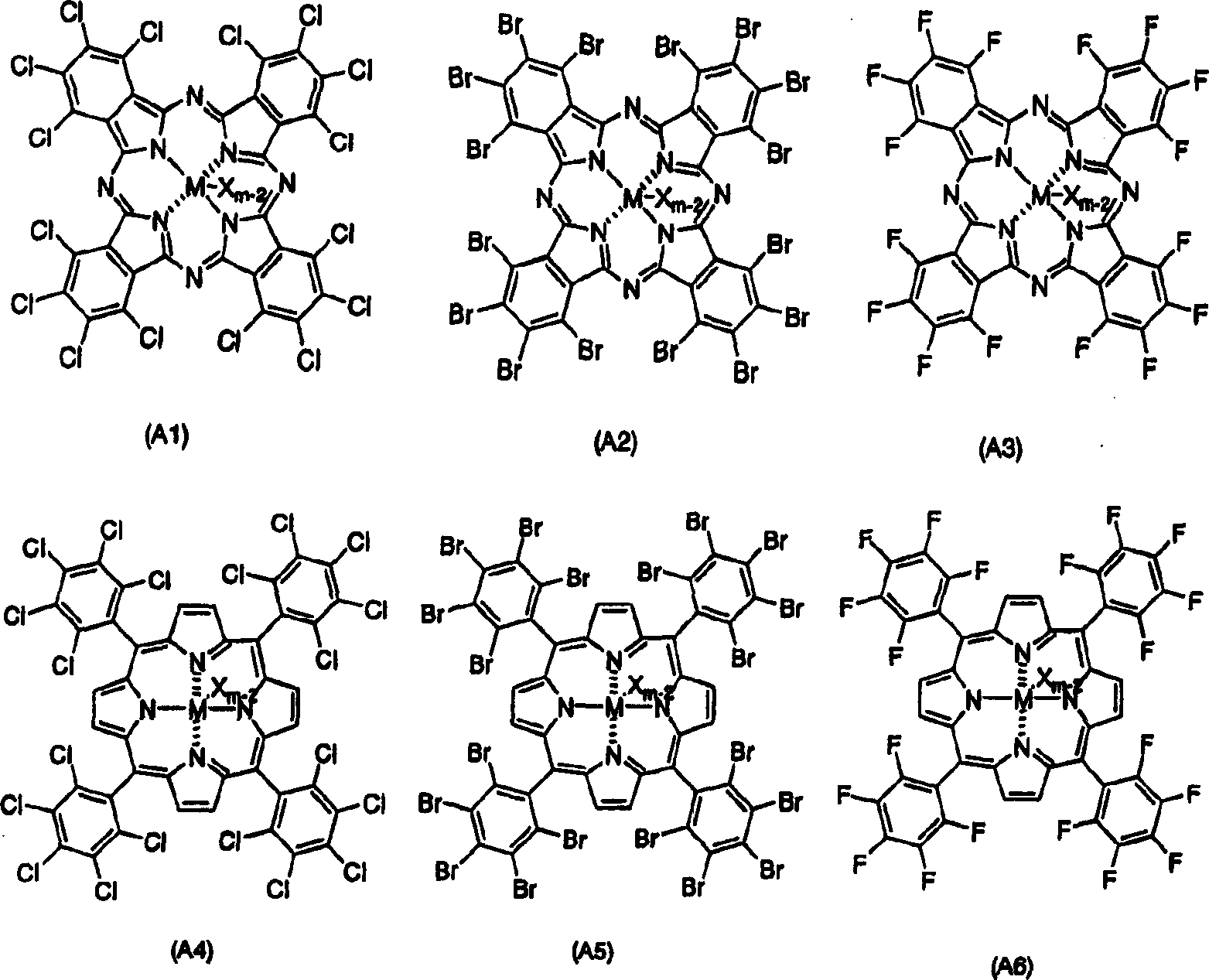
Component of polyaddition catalyst, polyaddition catalyst and process for preparing addition polymer
A technology of catalysts and compounds, applied in the field of preparation of addition polymers, which can solve problems such as undisclosed components
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0144] An autoclave having an inner volume of 400 ml equipped with a stirrer was dried under vacuum and replaced with argon, 190 ml of cyclohexane as a solvent and 10 ml of 1-hexene as a comonomer were added, and the autoclave was heated to 70°C . After heating, add ethylene, while adjusting the pressure of ethylene to 6kg / cm 2 . After the system is stable, add 0.25 mmol triisobutylaluminum, continuously add 1.0 μmol ethylene bis(indenyl) zirconium dichloride, then add 86.5 mg (101 μmol) of complex A with the following structure, and start polymerization . Polymerization was allowed to proceed for 30 minutes.
[0145] As a result of the polymerization, 18.6 g of an ethylene-1-hexene copolymer was obtained. The polymerization activity is 3.7×10 7 g / mol / h, SCB was 19.37, [η] was 1.29 dl / g, Mw was 88000, and Mw / Mn was 2.6.
[0146] Complex A:
[0147]
[0148] Manufactured by Aldrich Co., Ltd.
[0149] The orbital coefficient of the valence P-type atomic orbital is 0.8...
Embodiment 2
[0153] An autoclave having an inner volume of 400 ml equipped with a stirrer was dried under vacuum and replaced with argon, 190 ml of cyclohexane as a solvent and 10 ml of 1-hexene as a comonomer were added, and the autoclave was heated to 70°C . After heating, add ethylene, while adjusting the pressure of ethylene to 6kg / cm 2 . After the internal stabilization of the system, 0.25 mmol of triisobutylaluminum was added, and 77.1 mg (89.7 μmol) of complex A used in Example 1 was added continuously. After stirring for 30 minutes, 1.0 μmol of ethylenebis(indenyl)zirconium dichloride was added, and polymerization was carried out for 30 minutes.
[0154] As a result of the polymerization, 20.79 g of an ethylene-1-hexene copolymer was obtained. The polymerization activity is 4.2×10 7 g / mol / h.
Embodiment 3
[0156] Polymerization was performed in the same manner as in Example 1 except that 84.6 mg (97.7 μmol) of complex B having the following structure was used instead of complex A used in Example 1.
[0157] As a result, 2.46 g of an ethylene-1-hexene copolymer was obtained. The polymerization activity is 4.9×10 6 g / mol / h, [η] was 1.28dl / g, Mw was 73000, and Mw / Mn was 1.9.
[0158] Complex B:
[0159]
[0160] Manufactured by Aldrich Co., Ltd.
[0161] The orbital coefficient of the valence P-type atomic orbital is 0.773, and the orbital energy is 0.0049. Calculation was carried out in the same manner as complex A above.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com