Metabotropic glutamate receptor antagonists for treating central nervous system disease
A representative compound technology, applied in the field of metabolic glutamate receptor antagonists for the treatment of central nervous system diseases, can solve the problem of hindering mGluR physiological therapeutic efficacy, limited value, lack of effectiveness of mGluR agonists and antagonists and issues of selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
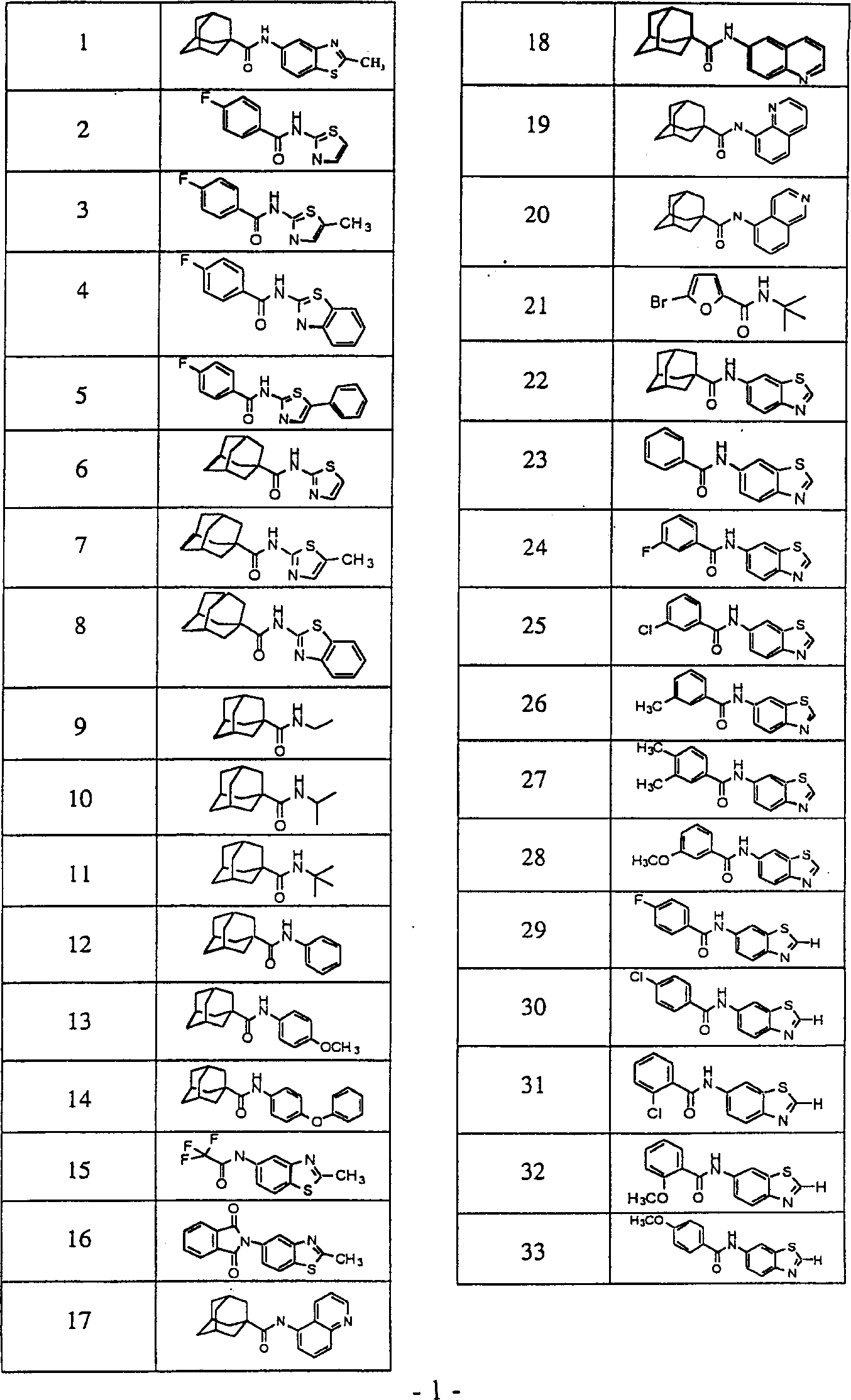
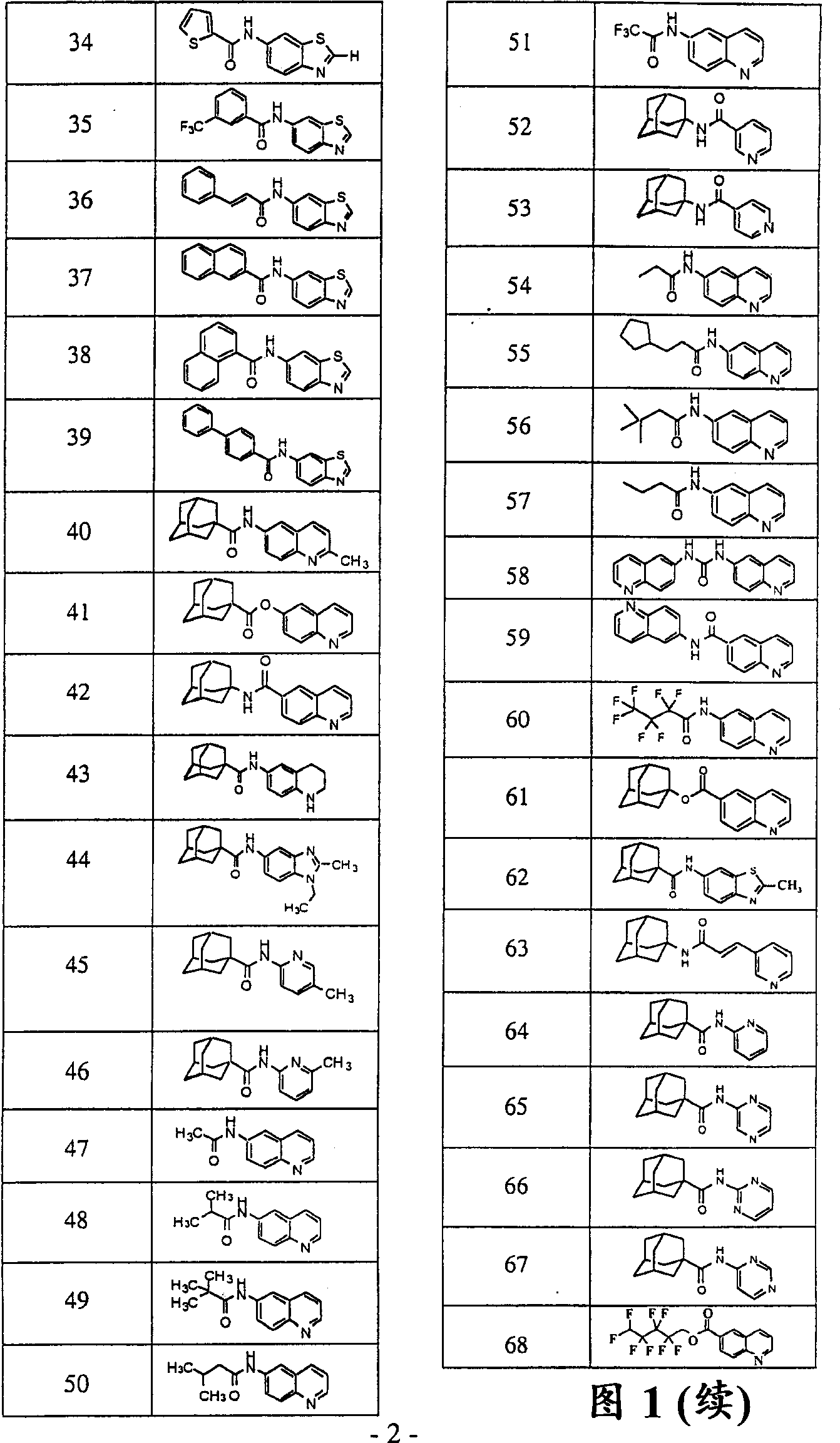
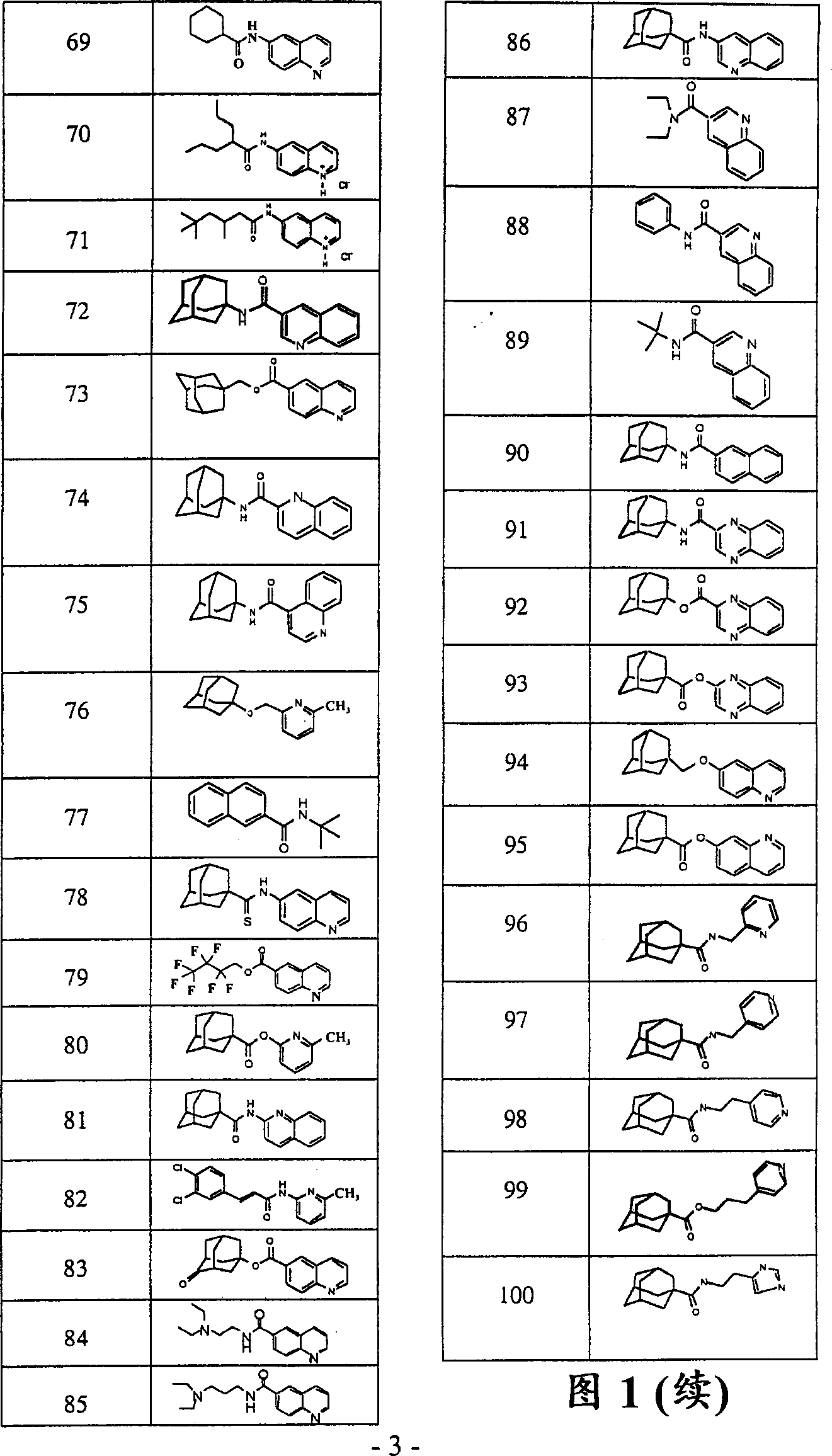
Image
Examples
Embodiment 1
[0102]Capillary gas chromatography and mass spectrometry data were measured by Hewlett-Packard (HP) 5890 II gas chromatograph, which was coupled with HP5971 Series Mass Selector Detector (mass selective detector) [Ultra-2 super performance capillary column (cross-linked with 5 %PhMe polysiloxane); column length, 25m; column inner diameter, 0.20mm; helium flow rate, 60mL / min; held at 325°C for 6 minutes]. Thin layer chromatography was performed on Analtech Uniplate 250 μm silica gel HF TLC plates. Compounds on TLC plates were assayed with UV light sometimes in combination with ninhydrin and Dragendorff developer (Sigma Chemical Co.). Reagents used were purchased from Aldrich Chemical Co. (Milwaukee, WI), Sigma Chemical Co. (SaintLouis, MO), Fluka Chemical Corp. (Milwaukee, WI), Fisher Scientific (Pittsburgh, PA), TCI America (Portland, OR) or Lancaster Synthesis (Windham, NH). Example 1: Preparation of N-[6-(2-methylquinolyl)]-1-adamantanecarboxamide (40)
[0103] 2-Methyl-...
Embodiment 2
[0118] 1-Adamantanecarbonyl chloride (1.37 g, 6.90 mmol) in pyridine (5 mL) was added to a solution of 6-hydroxyquinoline (1.00 g, 6.89 mmol) in pyridine (15 mL). The reaction was stirred for 16 hours. Water (200 mL) was added to the stirred reaction mixture causing precipitation of the product. The precipitate was filtered, washed with water (3 x 50 mL), and dried under high vacuum. 1.56g (73.7%) of (41) was obtained as light brown powder: retention time=11.41min.; m / z (relative intensity) 307(M+, 2), 136(11), 135(100), 116( 11), 107(7), 93(14), 92(2), 91(8), 89(7), 79(16), 77(8). Embodiment 3: Preparation of 6-quinolinecarboxylic acid 1- Adamantyl esters (61)
Embodiment 3
[0118] 1-Adamantanecarbonyl chloride (1.37 g, 6.90 mmol) in pyridine (5 mL) was added to a solution of 6-hydroxyquinoline (1.00 g, 6.89 mmol) in pyridine (15 mL). The reaction was stirred for 16 hours. Water (200 mL) was added to the stirred reaction mixture causing precipitation of the product. The precipitate was filtered, washed with water (3 x 50 mL), and dried under high vacuum. 1.56g (73.7%) of (41) was obtained as light brown powder: retention time=11.41min.; m / z (relative intensity) 307(M+, 2), 136(11), 135(100), 116( 11), 107(7), 93(14), 92(2), 91(8), 89(7), 79(16), 77(8). Embodiment 3: Preparation of 6-quinolinecarboxylic acid 1- Adamantyl esters (61)
[0119] 6-Quinolinecarbonyl chloride hydrochloride
[0120] In thionyl chloride, 6-quinolinecarboxylic acid was refluxed for 30 minutes. Then by rotary evaporation (90° C.), excess thionyl chloride was removed to obtain 6-quinolineformyl chloride hydrochloride.
[0121] 1-adamantyl 6-quinolinecarboxylate (61)
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com