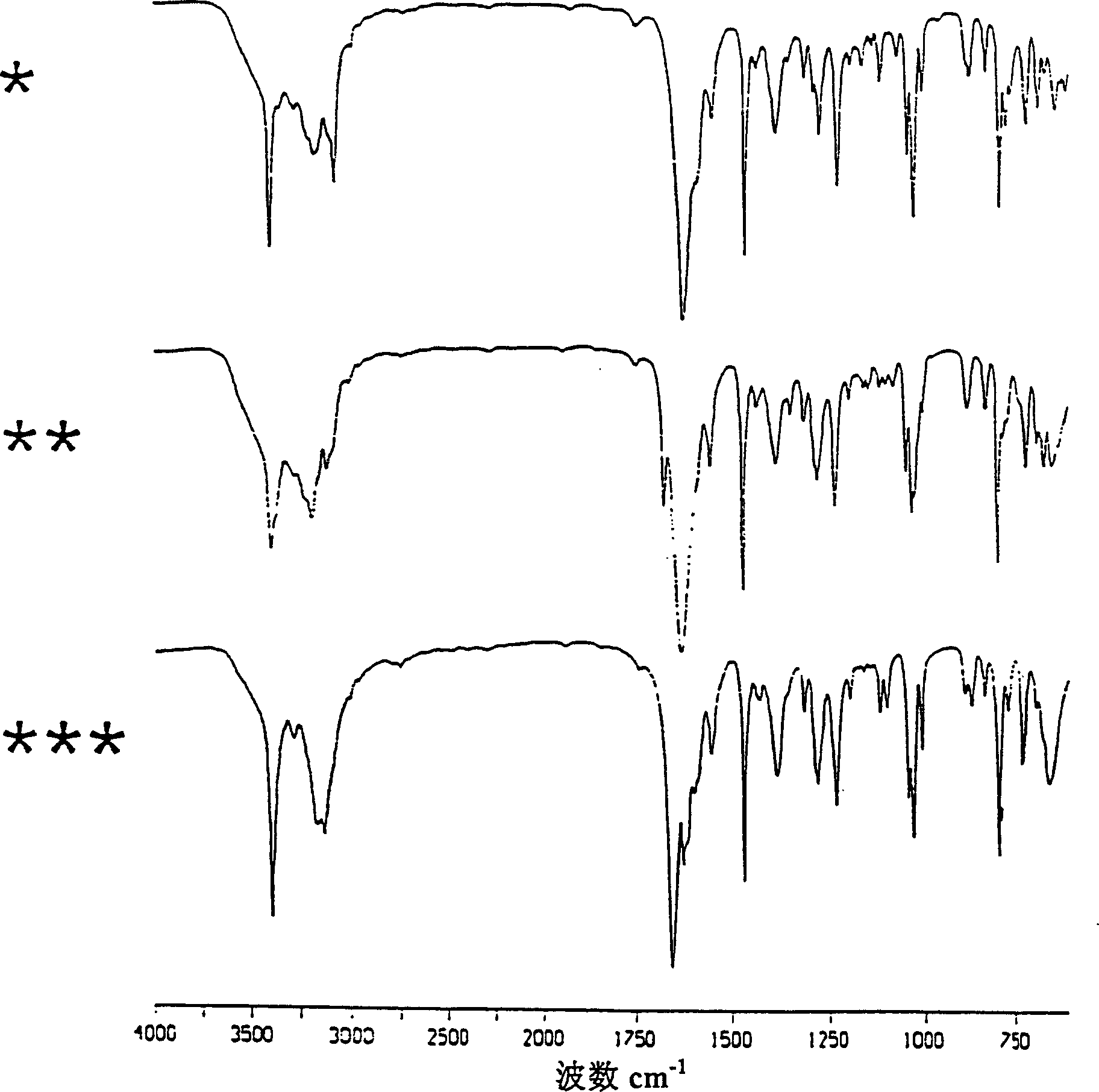
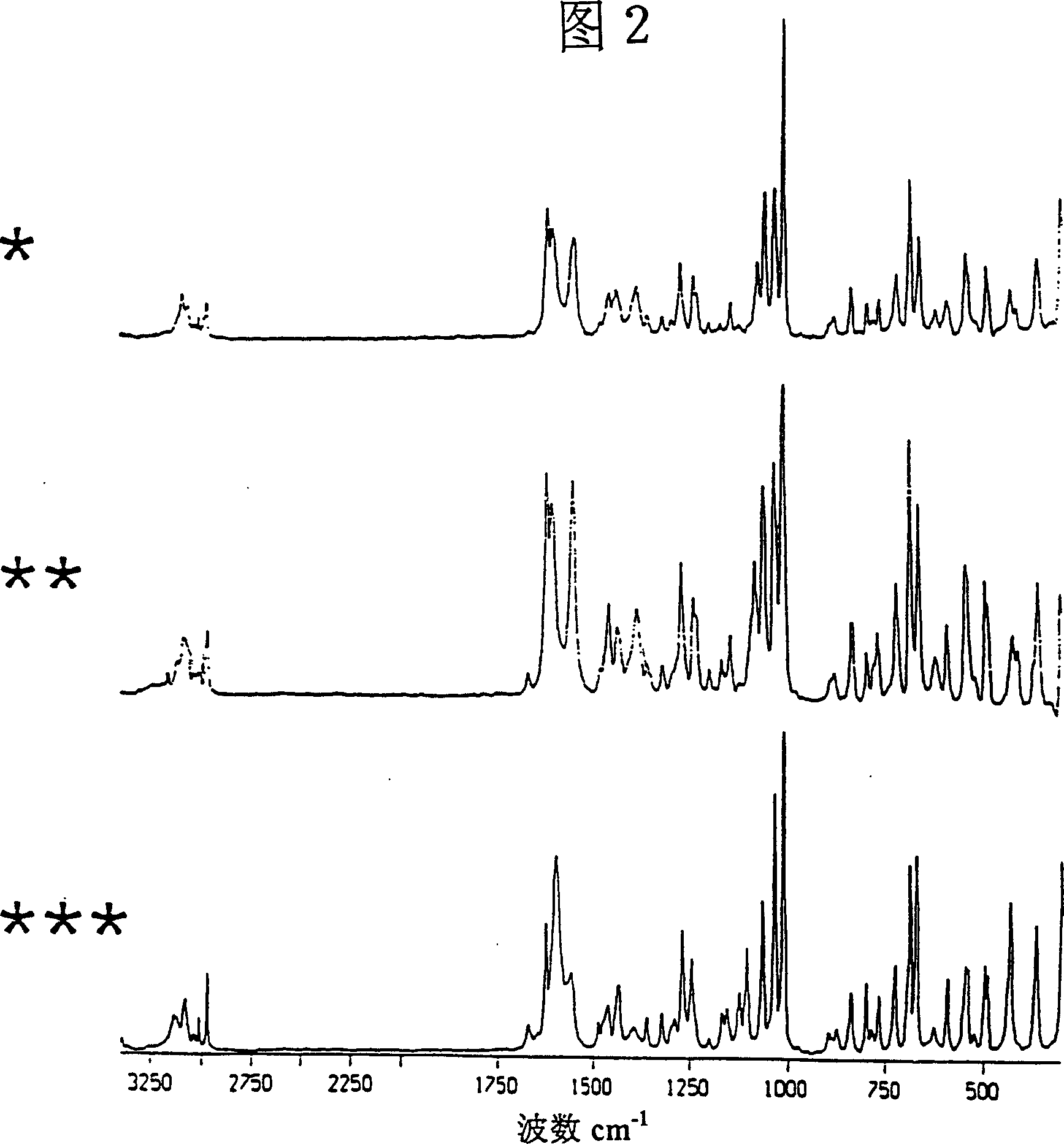
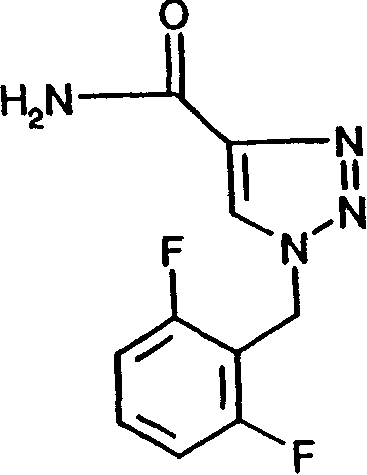
Use of certain crystal modifications of 1-(2,6-difluorobenzyl)-1h-1,2,3-triazole-4-carboxamide in treating epilepsy
A technology of difluorobenzyl and formamide, which is applied in the direction of medical preparations containing active ingredients, drug combinations, active ingredients of heterocyclic compounds, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Embodiment 1: Improved body B
[0076] 1-(2,6-Difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide (18.29 kg) was dissolved in formic acid (89.3 kg) with stirring at 58-63°C. The above solution was added to stirred methanol (105.5 L) at 20°C to 0°C over 30 minutes and washed with formic acid (6.1 kg) to form a suspension. The product was isolated immediately by filtration and washed with cold methanol (150 L, ca. 4°C). Vacuum drying at about 60°C yields improved product B with a yield of about 94%.
Embodiment 2
[0077] Embodiment 2: Improved Body C
[0078] 1-(2,6-Difluorobenzyl)-1H-1,2,3-triazole-4-carboxamide (15.0 g) was dissolved in acetic acid (120 ml) with stirring at about 90°C. The solution was cooled to 20°C over a period of about 8 minutes, forming a suspension. The product was immediately isolated by filtration, washed with toluene (120ml) and dried under vacuum at about 60°C. The improved product C was obtained. Yield 67.3%.
preparation Embodiment 1
[0081] The active ingredient is granulated together with deionized water. Ground lactose, corn starch, Avicel PH 102, Cellulose-HP-M-603 and sodium lauryl sulfate were then added to the above mixture and further granulated with deionized water.
[0082] The resulting wet material was dried and ground. After addition of the remaining ingredients the homogeneous mixture is compressed to obtain tablet cores having the above content of active ingredient.
[0083] The tablet cores are coated with a coating consisting of suitable ingredients dissolved or suspended in water or a small amount of ethanol containing 5% isopropanol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com