Biochemical material composition for preventing and treating disease caused by smooth muscle cell contraction in human organ
A technology of smooth muscle cells and biochemical substances, used in drug combinations, muscular system diseases, active ingredients of phosphorus compounds, etc., can solve the problems of patients with tinnitus that are not improved, and the cause and pathogenesis are not clear.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
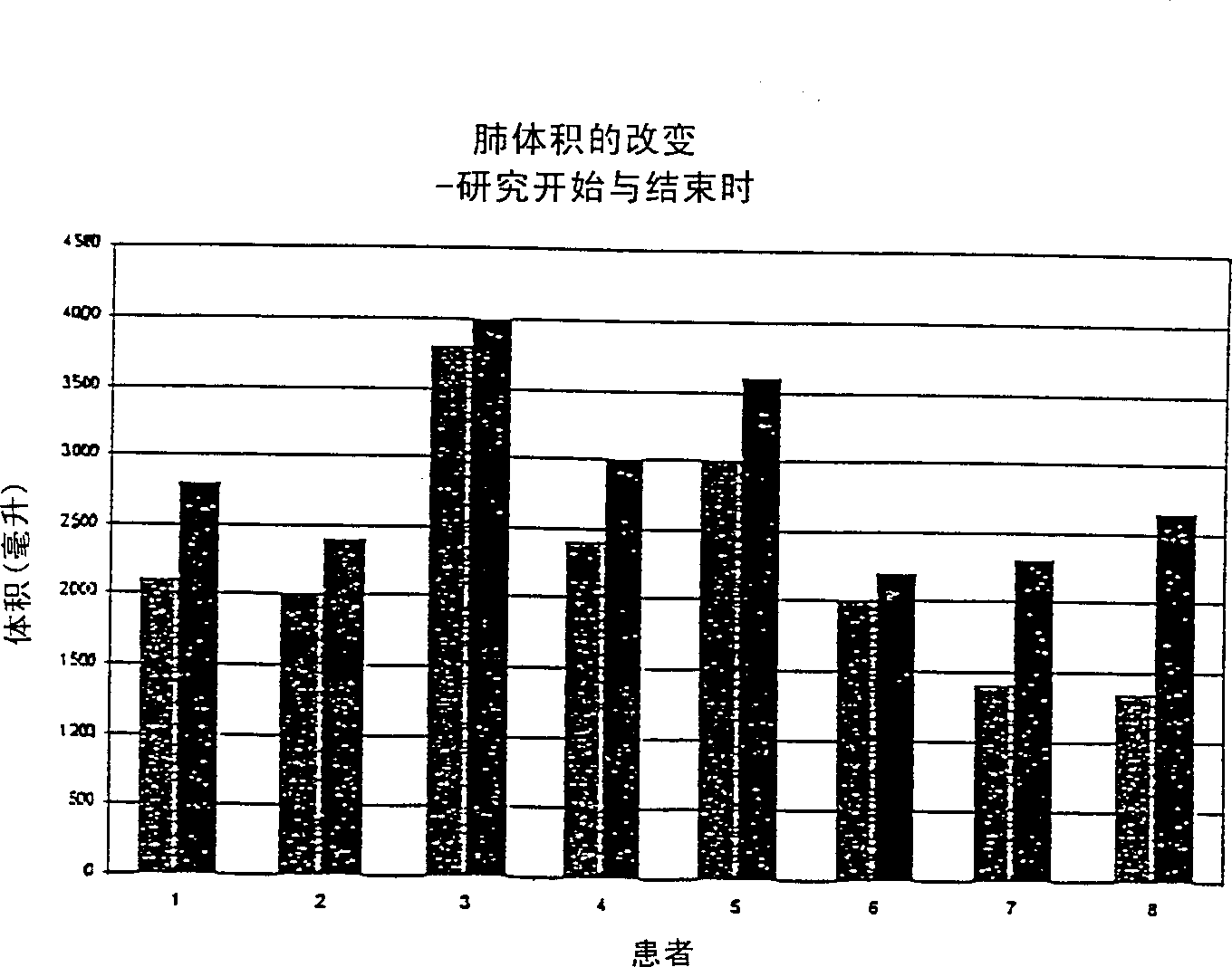
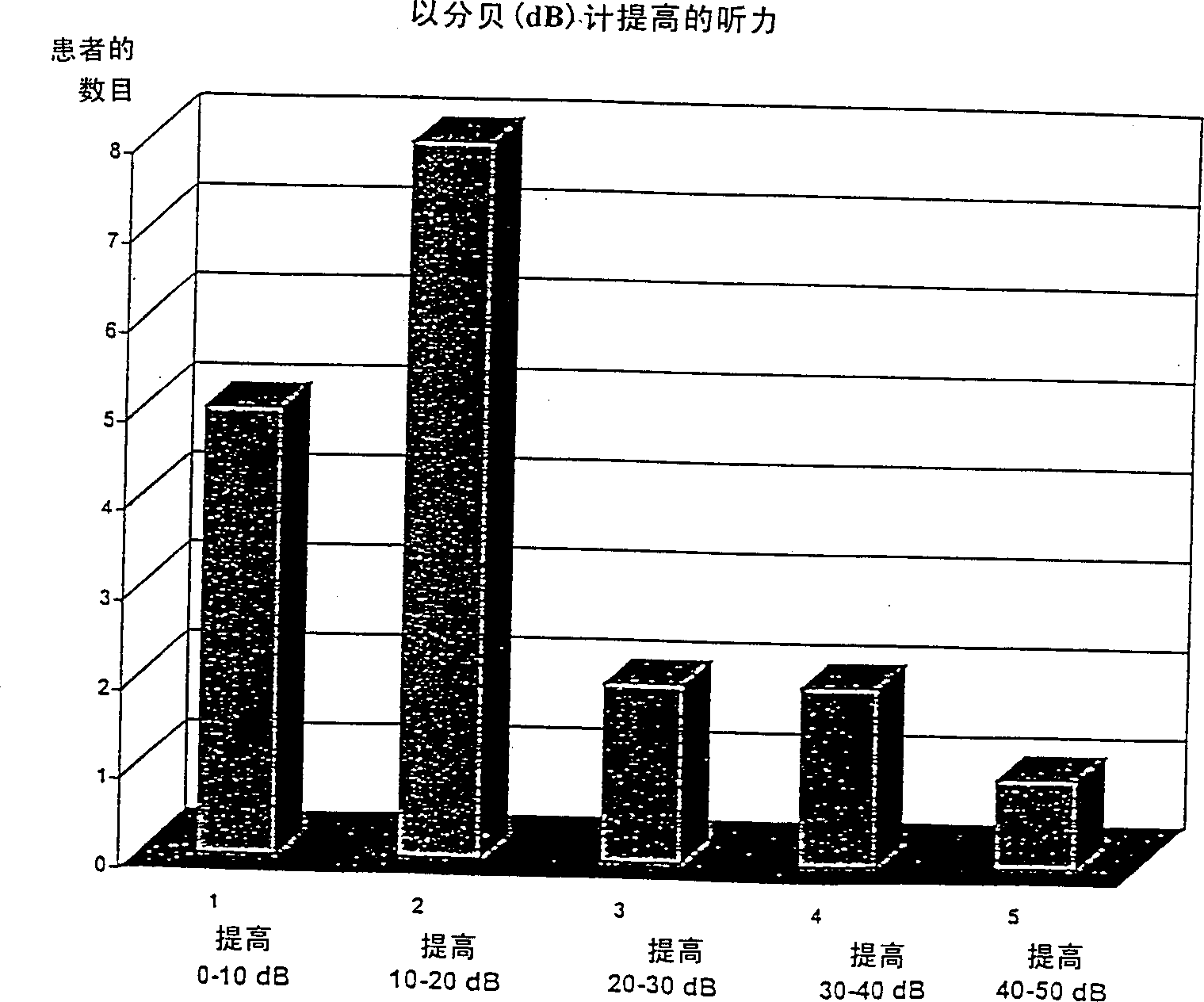
Image
Examples
Embodiment Construction
[0024] Compositions of the following biochemical substances were administered to the patient in daily unit amounts of the substances indicated in this example:
[0025] biochemical substance
unit
quantity
mg
680
Ascorbyl palmitate
mg
620
β-, γ-, δ-tocopherol mixture
mg
22
β-carotene
I.U.
1665
mcg
65
calcium ascorbate
mg
1050
Calcium Citrate
mg
200
calcium glycinate
mg
35
Carotenoid mixture (alpha-carotenoid
lutein, zea-, cryptoxanthin)
mcg
50
I.U.
130
Chromium Glycinate
mcg
10
Citrus Bioflavonoids
mg
650
Coenzyme Q10
mg
7
Copper Glycinate
mcg
330
mcg
20
d-alpha-tocopherol
I.U.
230
Calcium d-pant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com