Hepatocyte targeting polyethylene glyco-grafted poly-lysine polymeric gene carrier
A technology of polyethylene glycol and gene carrier, applied in gene therapy, genetic engineering, plant gene improvement, etc., can solve the problem of low transfection rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1L
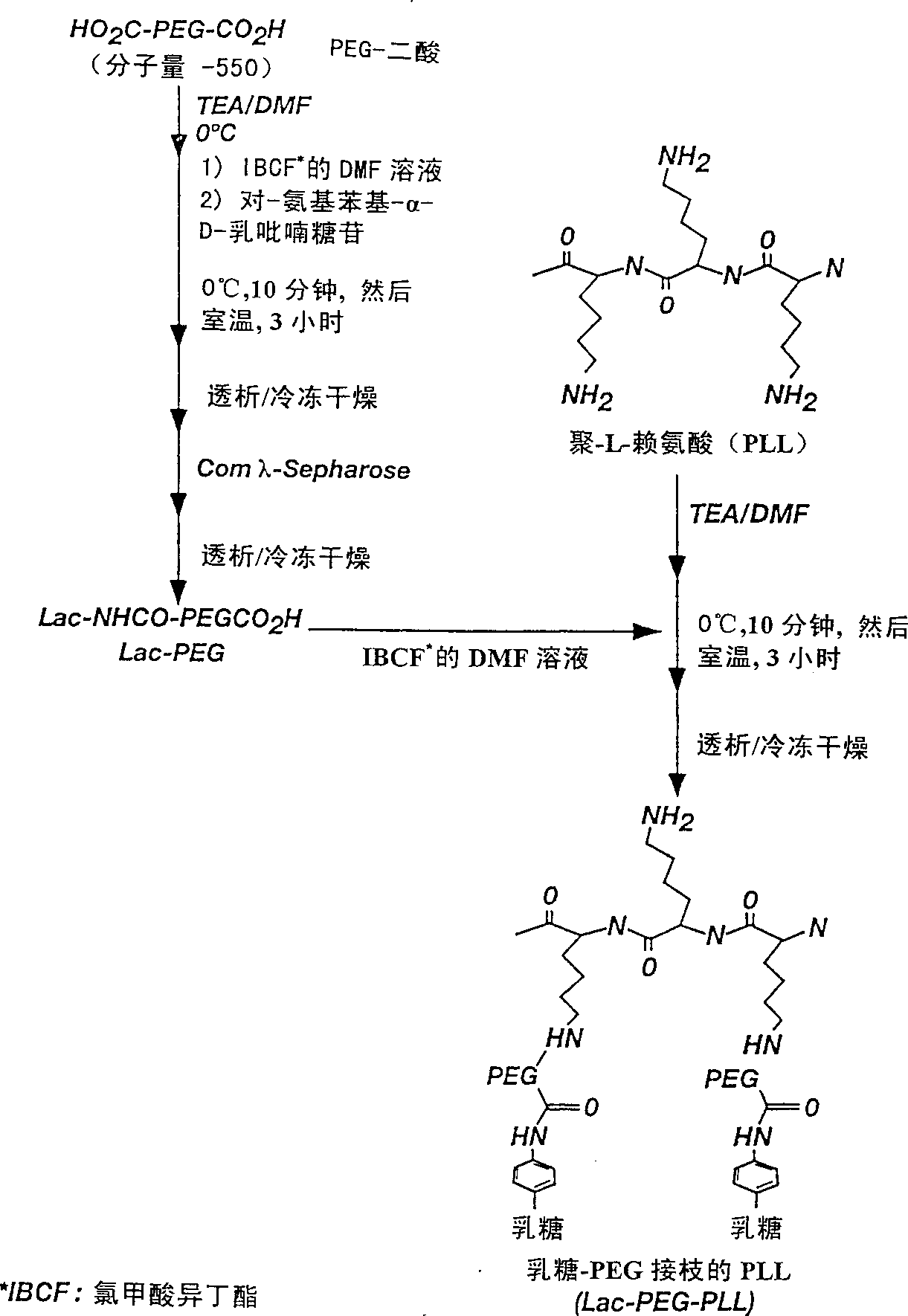
[0048] The synthesis of embodiment 1Lac-PEG diacid
[0049] The synthesis of lactose-PEG diacid was carried out as follows. Under a nitrogen atmosphere in an ice-water bath, 600 mg (1.0 mM) of PEG diacid (molecular weight = 600; Fluka, Ronkonkoma, NY) was dissolved in 1.5 mL of anhydrous N,N-Dimethylformamide (DMF; Aldrich). 1 mM isobutyl chloroformate (IBCF; Aldrich) dissolved in 1.0 mL of dry DMF was added dropwise in an ice-water bath, and the mixture was stirred for 15 minutes. IBCF acts as a carboxylic acid activator. Then p-aminophenyl-α-D-lactopyranoside (1.0 mM; Sigma) dissolved in 1.0 mL of DMF was added, and the mixture was stirred in an ice-water bath for 30 minutes, then at room temperature for a further 3 hours. The product was precipitated in excess dry diethyl ether (25 mL). The precipitate was then dissolved in 20 mL of distilled water, dialyzed against distilled water (MWCO of the dialysis bag was 1,000; Spectrum, Houston, TX) and lyo...
Embodiment 2
[0050] The grafting of embodiment 2 lactose-PEG diacid and PLL
[0051] In this example the synthesis of Lac-PEG-PLL with 30% (mol / mol%) lactose-PEG) is described. Lactose-PEG and lactose-PEG-lactose mixture (51.5 mg; containing 0.042M lactose-PEG) prepared according to the procedure of Example 1 were dissolved in 0.5 mL of anhydrous DMF under a nitrogen atmosphere in an ice-water bath. IBCF (0.036M) dissolved in 0.5 mL DMF was added dropwise to the above solution. The mixture was stirred in an ice-water bath for 30 minutes, then added dropwise to 1 mL of anhydrous dimethylsulfoxide (DMSO; Aldrich) containing 25 mg of PLL-HBr (120 repeating units; molecular weight = 25,000; Sigma) and 10 μLTEA (which had been purged with nitrogen in an ice-water bath for 10 minutes), then stirred at room temperature for an additional 3 hours. The product was precipitated in 100 mL of anhydrous ether, and the precipitate was dissolved in 10 mL of distilled water. Disso...
Embodiment 3
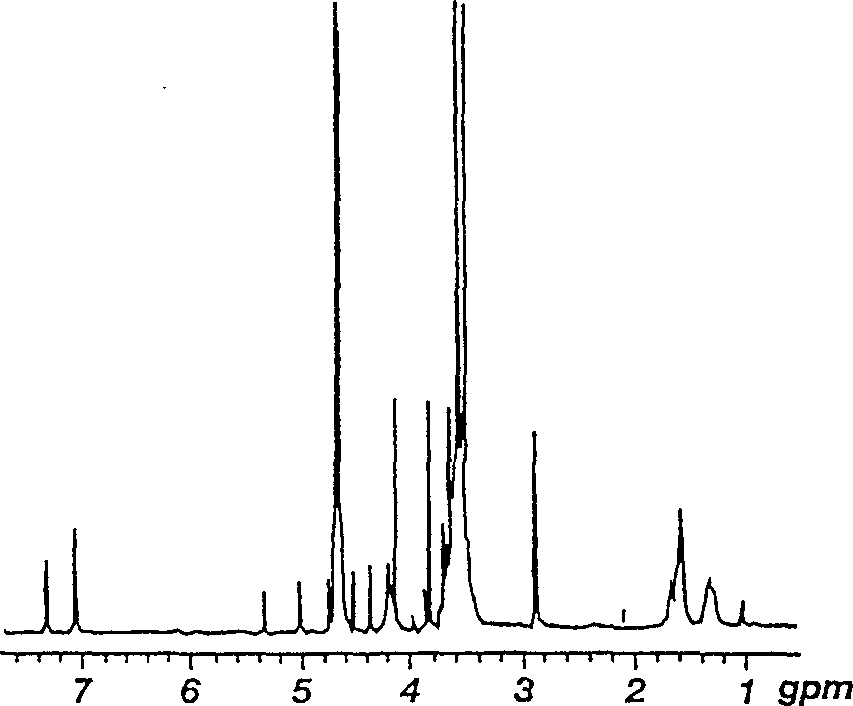
[0052] The 1H-NMR analysis of embodiment 3 lactose-PEG-PLL
[0053] 1H-NMR analysis of Lac-PEG-PLL prepared by the procedures of Examples 1 and 2 was carried out in water. exist figure 2 The 1H-NMR spectrum of 30 mol% Lac-PEG-PLL is shown in . A peak at approximately 3.5 ppm indicates the presence of PEG in the composition. The PEG content was calculated from the NMR spectrum by correlating the PEG (-CH2CH2-.s.3.4-3.7ppm) peak with the PLL side chain (-CH2CH2CH2-.m.1.1-1.8ppm) peak. The following ratios of lactose-PEG content in four illustrative carrier compositions made by the present invention were confirmed by this method: 6, 12, 20 and 30 mole %, respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com