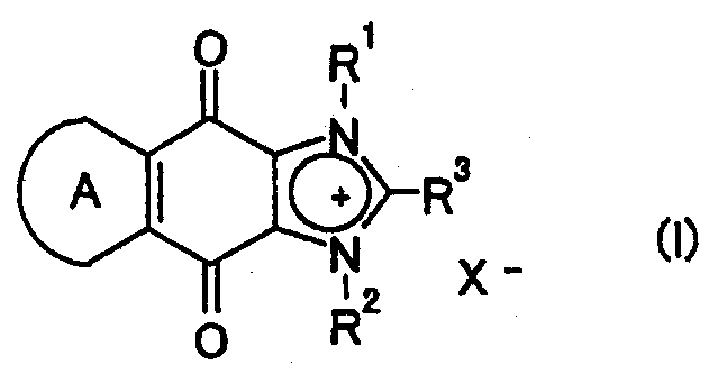
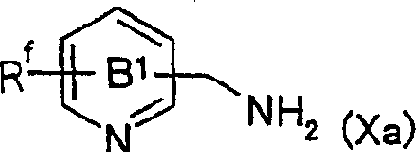
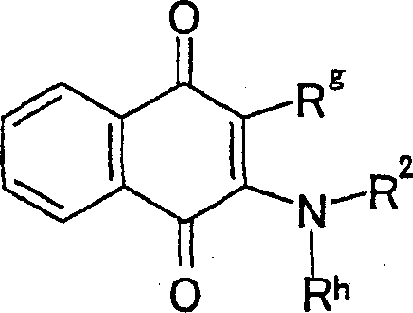
Fused imidazolium derivatives
A technology of imidazolium and derivatives, applied in the field of synthetic intermediate compounds, can solve the problems of undisclosed compound drug use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
reference example 1
[0132] Reference example 1: in the ethanol (50 milliliters) solution of 3-cyano-2-(dimethylamino) pyridine (2.45 grams), add saturated ammonia (17 milliliters) and Raney nickel (3.0 grams), in 1 Stir at room temperature under a hydrogen atmosphere of 1 atm for 8 hours. After 760 ml of hydrogen uptake, the catalyst was removed by filtration. The mother liquor was concentrated to give 3-(aminomethyl)-2-(dimethylamino)pyridine (2.61 g) as a yellow oil.
reference example 2
[0133] Reference example 2: in acetic anhydride (100 milliliters) solution of 2-chloro-3-[(2-methoxyethyl) amino]-1,4-naphthoquinone (33 grams), add several drops of concentrated sulfuric acid, Stir at 45°C for 1 hour. Ethanol (100 ml) was added to the reaction solution to esterify excess acetic anhydride. After cooling, ethyl acetate was added, washed with water and saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed by evaporation, and the residue was crystallized from ether to give N-(3-chloro-1,4-dihydro-1,4-dioxo-2-naphthyl)-N-(2-methoxy ethyl)acetamide (29 g).
reference example 3
[0134] Reference Example 3: In N-(3-chloro-1,4-dihydro-1,4-dioxo-2-naphthyl)acetamide (1.0 g) in benzene (20 ml) solution, add 2-form Oxyethylamine (0.8 mL), stirred at room temperature for 1 hour. Water was added to the reaction liquid, followed by extraction with chloroform. The organic layer was washed with water and saturated brine, and dried over anhydrous sodium sulfate. The solvent was removed by evaporation, and the residue was recrystallized from ethyl acetate to give N-[3-(2-methoxyethyl)amino-1,4-dihydro-1,4-dioxo-2-naphthalene as a red powder base] acetamide (0.87 g).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com