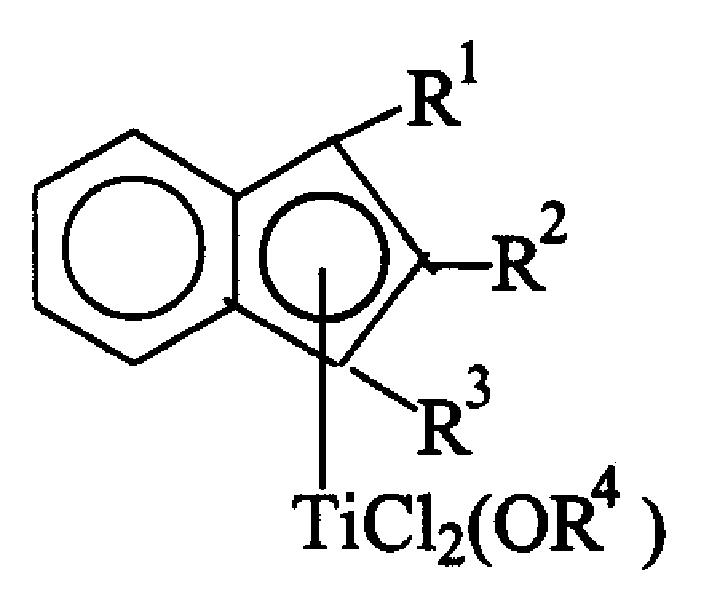
Substituted indenyl metal titanium compound and its preparation method and use
A technology of titanium compounds and base metals, applied in the direction of titanium organic compounds, etc., can solve the problem of low catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0033] All operations are the same as in Example 1, except that methanol is used instead of ethanol to finally obtain 1-CH 3 IndTiCl 2 (OMe) red crystals, yield 73.6%, m.p.=110-115°C. The analysis test results are as follows
[0034] Molecular formula: C 11 h 12 Cl 2 OTi;
[0035] M.S. (EI): 278 (6, M + ).
[0036] 1 H NMR (CDCl 3 , δ): 7.76~7.68 (m, 2H, IndH 2 ), 7.47~7.44 (m, 2H, IndH 2 ), 6.72 (d, IH, J=3.3, IndH), 6.61 (d, 1H, J=3.3, IndH), 4.36 (s, 3H, OCH 3 ), 2.64(s, 3H, IndH 3 )
[0037] E.A.: Measured, C: 52.37% H: 5.65%
Embodiment 3
[0039] All operations are the same as in Example 1, except that isopropanol is used instead of ethanol to finally obtain 1-CH 3 IndTiCl 2 (O i Pr) Red crystals, yield 32.3%, m.p.=59-61°C. The analysis test results are as follows
[0040] Molecular formula: C 13 h 16 Cl 2 OTi;
[0041] M.S. (EI): 306 (6, M + ).
[0042] 1 H NMR (CDCl 3 , δ): 7.75~7.73 (m, 2H, IndH 2 ), 7.45~7.41 (m, 2H, IndH 2 ), 6.68 (d, 1H, J=3.3, IndH), 6.57 (d, 1H, J=3.3, IndH), 4.97 (m, 1H, J=6.1, OCH (CH 3 ) 2 ), 2.63(s, 3H, IndH 3 ), 1.31 (d, 6H, J=6.1, OCH (CH 3 ) 2 )
[0043] E.A.: Measured, C: 50.87% H: 5.25%
Embodiment 4
[0045] All operations are the same as in Example 1, except that ethanol is replaced by cyclohexyl, and finally 1-CH is obtained 3 IndTiCl 2 Red crystals, yield 37.3%, m.p.=84-86°C. The analysis test results are as follows
[0046] Molecular formula: C 16 h 20 Cl 2 OTi;
[0047] M.S. (EI): 346 (1, M + ).
[0048] 1 H NMR (CDCl 3 , δ): 7.76~7.73 (m, 2H, IndH 2 ), 7.48~7.42 (m, 2H, IndH 2 ), 6.68(d, 1H, J=3.3, IndH), 6.58(d, 1H, J=3.3, IndH), 4.75(m, 1H, ), 2.63(s, 3H, IndH 3 ), 1.90~1.28(m, 10H, )
[0049] E.A.: Measured, C: 55.36% H: 5.81%
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com