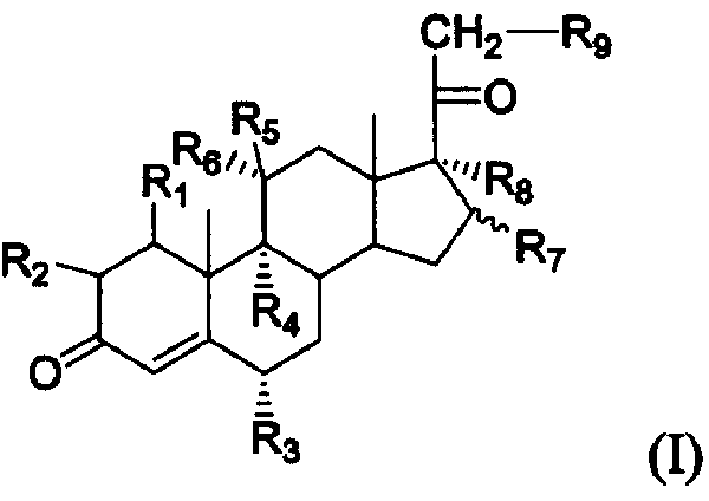
Steroid compound containing nitrogen and its preparation method
A technology of steroidal compounds and compounds, which is applied in the field of glucocorticoid nitrogen-containing steroidal compounds and their preparation, and the intermediates for the preparation of these compounds, and can solve problems such as not much progress in research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Preparation of 11β,17α,21-trihydroxy-pregn-4-ene-3,20-dione-21-methanesulfonate
[0028] 11β, 17α, 21-trihydroxy-pregn-4-ene-3,20-dione (hydrocortisone, 30g; 82.77mmol), pyridine (160ml) in a 250ml three-necked flask, cooled to 0°C, To this was added methanesulfonyl chloride (15.5ml, 191.64mmol) dropwise over half an hour. React at 0-5°C for 3 hours. After the reaction, pour the reaction solution into 2000ml of ice water, let it stand for more than 2 hours, filter with suction, wash with water, and dry to obtain 33.53g of crude product with a yield of 91.95%.
[0029] The obtained crude product was recrystallized from a mixed solvent of chloroform / methanol to obtain 30.5 g of pure product. Mp178~180(dec.)℃.
[0030] Elemental Analysis: C 22 h 32 o 7 S, Cal.C59.98%, H7.32%; Found: 59.88%, H7.50%
[0031] 1 HNMR (δ, 200MHz, DMSO-d6): 5.55(1H, s), 5.49(1H, s), 5.28(1H, d, J AB 17.6Hz), 4.87 (1H, d, J AB 17.6Hz), 4.35(1H, d, J3.1Hz), 4.25(1H, s), 3.23(3H, s), 1.35(...
Embodiment 2
[0033] Preparation of 11β, 17α, 21-trihydroxy-pregna-1,4-ene-3,20-dione-21-methanesulfonate
[0034]11β, 17α, 21-trihydroxy-pregna-1,4-en-3,20-one (prednisolone, 50.33g, 139.63mmol), tetrahydrofuran (500ml), triethylamine (46.70ml, 335.11mmol ) in a 1000ml three-neck flask, stirred mechanically, added methanesulfonyl chloride (25.5ml, 329.46mmol) dropwise at below 0°C within 0.5 hours, and reacted at 0-5°C for 3 hours. After the reaction, the reaction solution was extracted with 1000ml of ethyl acetate, washed with 300ml×3 water, the organic phase was taken, dried over anhydrous sodium sulfate, and then concentrated to dryness under reduced pressure to obtain 58.2g of crude product with a yield of 95.04%. Mp199~200℃.
[0035] 1 HNMR (δ, 200MHz, DMSO-d6): 7.33(d, 1H, J9.9Hz), 6.17(d, 1H, J9.9Hz), 5.90(s, 1H), 5.5(s, 1H), 5.27(d ,1H,J AB 17.5Hz), 4.91 (d, 1H, J AB 17.5Hz), 4.8(s, 1H), 4.27(s, 1H), 3.22(s, 3H), 1.37(s, 3H), 0.79(s, 3H)
[0036] [Ref.Krajcsi, P.and Arányi, P...
Embodiment 3
[0038] Preparation of 11β,17α,21-trihydroxy-6α-methyl-pregna-1,4-diene-3,20-dione-21-methanesulfonate
[0039] 11β, 17α, 21-trihydroxy-6α-methyl-pregna-1,4-diene-3,20-dione (methylprednisolone, 50g, 133.52mmol), with methanesulfonyl chloride (23.5ml, 303.62 mmol) was esterified to obtain 58.87 g of methanesulfonate, with a yield of 97.42%. Get 1g crude product, through flash column chromatography (CH 2 Cl 2 :CH 3 OH=10:0.2), yielding 0.9 g of analytical sample. Mp199~201℃.
[0040] 1 HNMR (δppm, DMSO-d6): 7.32 (d, 1H, J10.21Hz, C 1 -H), 6.17(d, 1H, J9.91Hz, C 2 -H), 5.81(s, 1H, C 4 -H), 5.48(s, 1H, C 11 -OH), 5.26(d, 1H, J17.8Hz, C 21 -H), 4.87(d, 1H, J17.6Hz, C 21 -H), 4.69(s, 1H, C 17 -OH), 4.27(s, 1H, C 11 -H), 3.23(s, 1H, SO 3 -CH 3 ), 1.37 (s, 3H, C 19 -CH 3 ), 1.04(d, 1H, J6.0Hz, C 6 -CH 3 ), 0.80(s, 1H, C 18 -CH 3 )
[0041] HRFAB (M+1): C 23 h 32 o 7 The theoretical value of S is 453.1941, and the measured value is 453.1949.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mp | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com